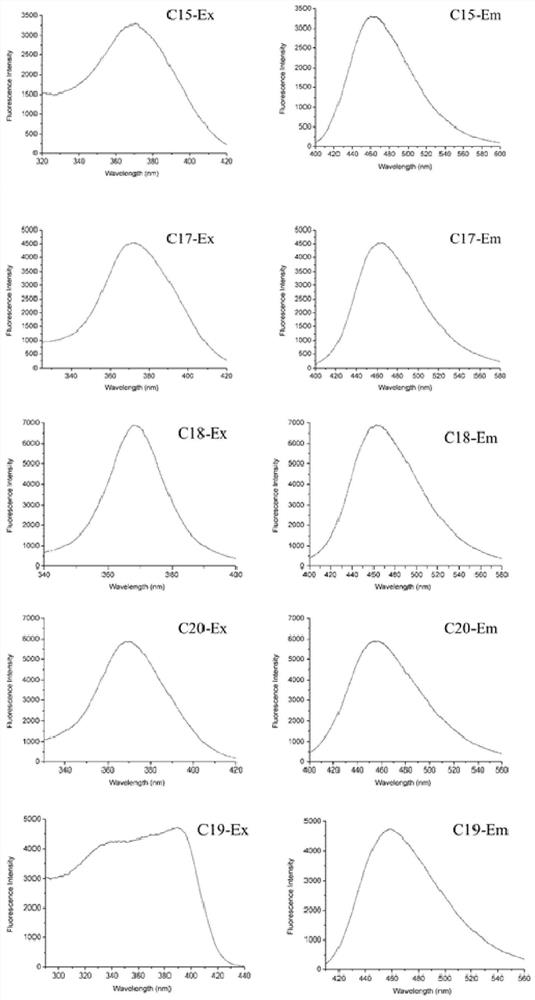
Furocoumarin compound and application thereof in detection of mycobacterium tuberculosis
A technology of mycobacterium tuberculosis and compounds, applied in organic chemistry, sugar derivatives, analytical materials, etc., can solve problems such as low signal-to-noise ratio and instability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
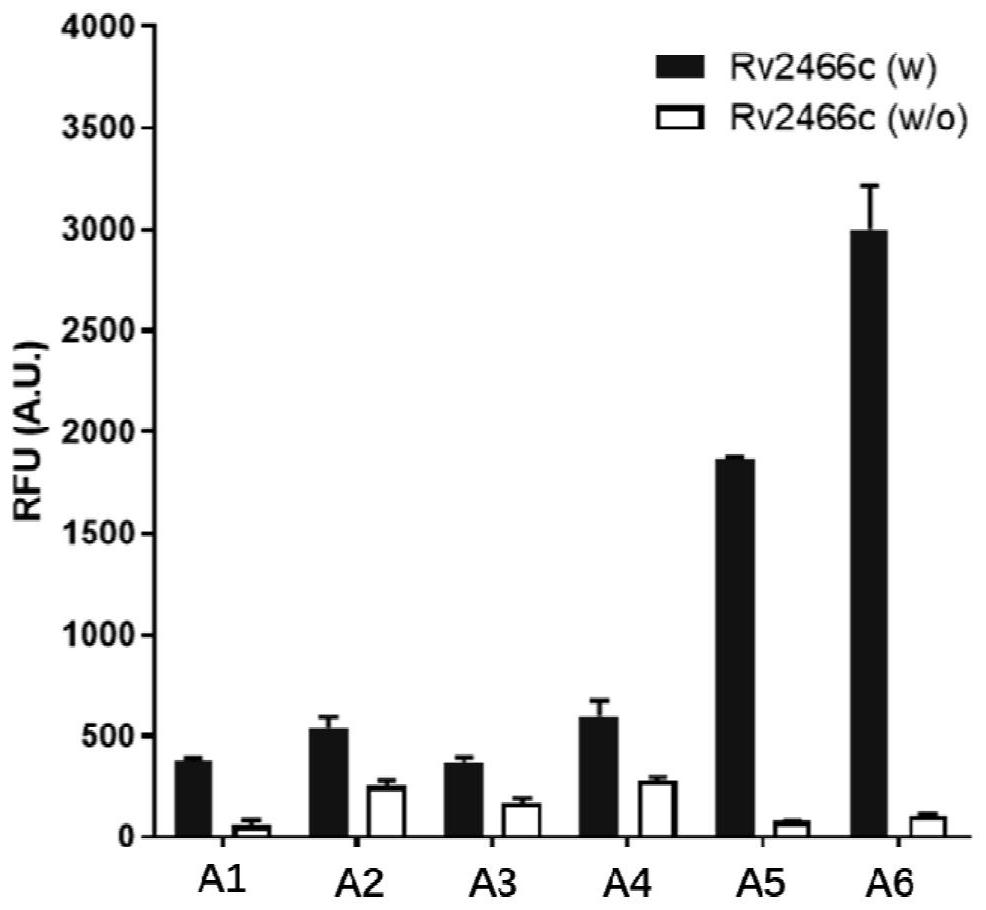
[0084] Embodiment 1 The fluorescence response of the compound of the present invention to Rv2466c enzyme
[0085] Instrument: multifunctional microplate reader ( Flash, Thermo Scientific).
[0086] Reagents and materials: compound (A1-A6), Rv2466c, MSH, Tris-Cl (pH 7.5), DMSO, 96-well microtiter plate (Corning, 3603).
[0087] Operation: 1) Prepare 50mM Tris-Cl (pH 7.5) buffer solution containing 0.5mM MSH, and put 20mL buffer solution into two sterile tubes. 2) Add a certain amount of Rv2466c to one of the tubes so that the final concentration is 200 μg / mL, which is the enzyme-containing working solution, and add the same amount of Tris-Cl buffer solution to the other tube as the enzyme-free control solution. 3) The compounds of the present invention were respectively dissolved in DMSO to prepare a solution of 25 μg / mL, and 20 μL of each compound was added to four wells of a 96-well plate, two of which were used as a control group without enzymes , and the other two wells...
Embodiment 2
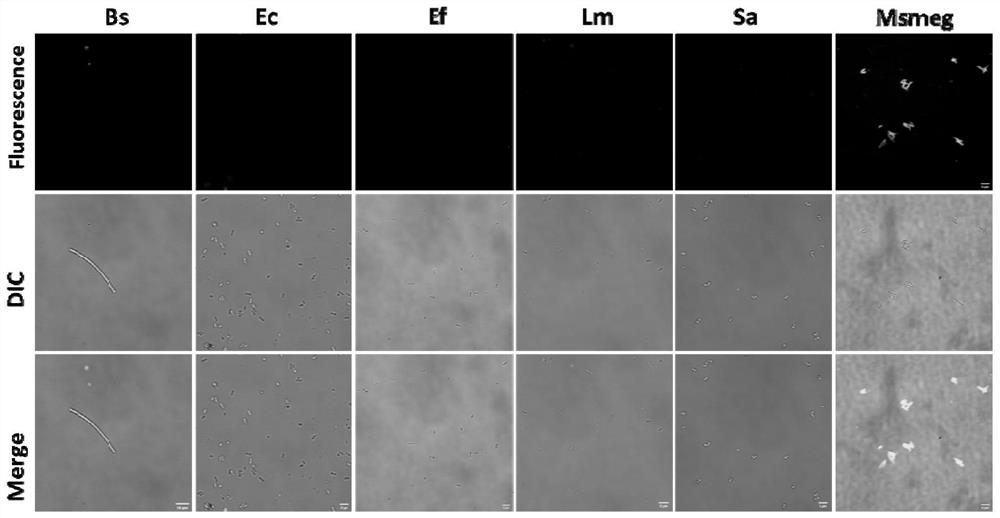
[0089] Embodiment 2 The specificity experiment of compound A6 of the present invention
[0090] Instrument: Fluorescence microscope (Delta Vision high-resolution live cell imaging system).
[0091] Reagents and materials: compound A6, intermediate 12, Bacillus subtilis (Bs), Escherichia coli (Ec), Enterococcus faecalis (Ef), Listeria monocytogenes (Lm), Staphylococcusaureus (Sa), Mycobacterium smegmatis (M.smeg), cultured in LB base, THY medium, 7H9 medium.
[0092] Operation: Prepare the compound A6 and intermediate 12 at a concentration of 10 mM. After the bacteria are cultured to the logarithmic growth phase, take 200 μL of bacterial liquid and add 2 μL of bacterial liquid to 2 μL A6 and intermediate 12 respectively. After incubating at 37°C for 30 minutes, prepare a slide sample and examine it under a fluorescence microscope. observe. The results show that the compound of the present invention has specificity to mycobacteria, and does not stain to other Gram-negative bac...
Embodiment 3
[0094] Embodiment 3 The dyeing ability of compound A6 of the present invention to Mtb
[0095] Instrument: fluorescence microscope.
[0096] Reagents and materials: Compounds A1 and A6, Mycobacterium tuberculosis (Mtb), 7H9 medium.
[0097] Operation: Prepare the compounds A1 and A6 at a concentration of 10 mM respectively, culture the bacteria to the logarithmic growth phase, take 200 μL of the bacterial liquid and add ebselen (Eb, the final concentration is 50 μg / mL), incubate for 3 hours and centrifuge to remove ebselen, respectively. Add 200 μL 7H9 medium and 2 μL A1, A6, and incubate at 37°C for 30 minutes, prepare slide samples and observe under a fluorescence microscope. The results are as follows: Figure 5 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com