Novel genipin derivatives and preparation method and application thereof
A technology of genipin and derivatives, which is applied in the field of preparation of new genipin derivatives, and can solve problems such as lack of Scp1 inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
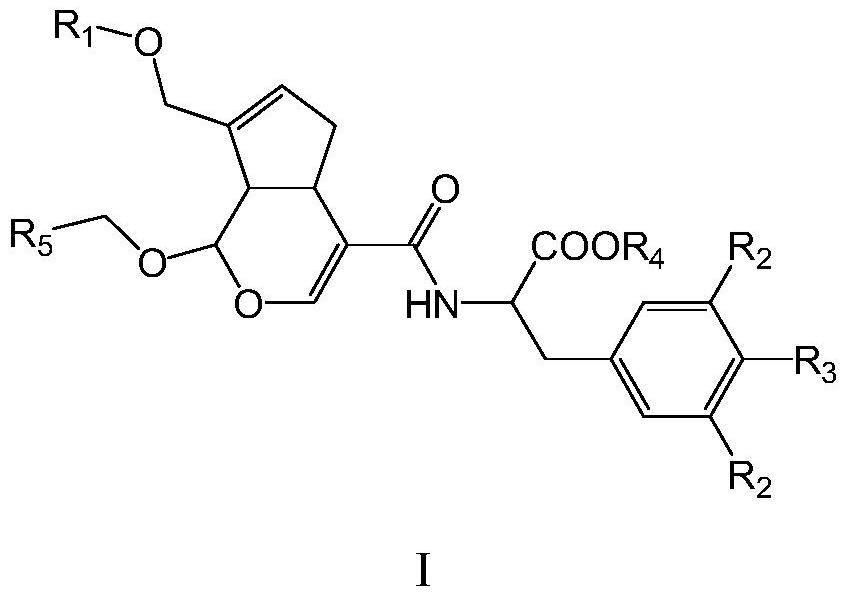
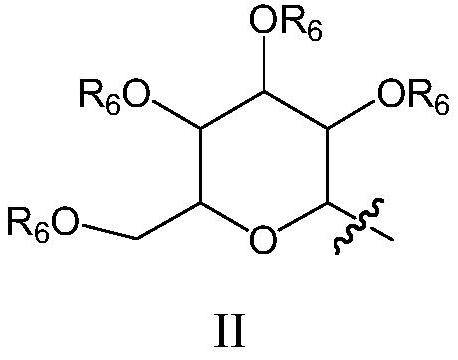
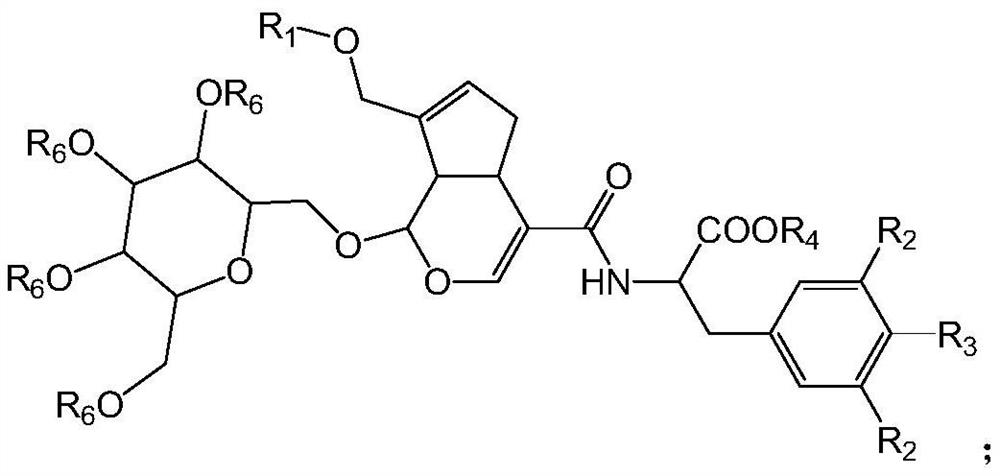
[0081] The invention provides a preparation method and application of novel genipin derivatives. Based on the structural characteristics of Scp1, the present invention designs novel genipin derivatives. There is a hydrophobic end in its structure, and there is a carboxylic acid group as the hydrophilic end, which are respectively larger than the hydrophobic pocket of Scp1 and the magnesium ion in the active center and the surrounding magnesium ions. Hydrophilic pocket binding. The exposed carboxyl group in the molecule can form intermolecular hydrogen bonds with Ser211, Asp206, Lys190 or Asp96 in the Scp1 pocket, and the benzyl group in the side chain can form π with any or more residues in Phe106, Tyr188, and Tyr158 in the active pocket -π interaction, improving the stability of small molecules and protein binding. The evaluation of Scp1 inhibitory activity shows that the novel compound prepared by the present invention has good Scp1 inhibitory activity and can be used to pr...
Embodiment 1
[0145] The synthesis of embodiment 1 compound III
[0146]
[0147] Dissolve geniposide (1g, 2.58mmol) in DMF (10mL), slowly add 60% sodium hydride (0.618g, 15.46mmol) under ice-water bath, after reacting for 1h, slowly add benzyl bromide (15.46mmol), and React overnight. After the reaction, the reaction solution was added to ice water (60mL), extracted three times with ethyl acetate (90mL), the organic phases were combined, washed three times with 1M hydrochloric acid solution (30mL), saturated brine (60mL) successively, and anhydrous Dry over sodium sulfate, concentrate under reduced pressure, and separate and purify by silica gel chromatography to obtain light yellow oily liquid with a yield of 70%. 1 H NMR (400MHz, Chloroform-d) δ7.54(s, 1H), 7.38(d, J=4.2Hz, 1H), 7.33(q, J=5.3, 4.6Hz, 22H), 7.22(d, J= 7.2Hz, 2H), 5.87(s, 1H), 5.36(d, J=5.8Hz, 1H), 5.29(s, 1H), 4.95(t, J=10.7Hz, 2H), 4.87(d, J= 9.2Hz, 2H), 4.70–4.50(m, 7H), 4.22(d, J=13.7Hz, 2H), 3.75(d, J=11.1Hz, 1...
Embodiment 2
[0149]
[0150] Referring to Example 1, a light yellow oily liquid was obtained with a yield of 61%. 1H NMR (400MHz, Chloroform-d) δ7.60(s,1H),7.35–7.10(m,20H),5.91(s,1H),5.42(s,1H),4.96(s,1H),4.90( dd,J=12.2,3.0Hz,2H),4.85(s,1H),4.75–4.47(m,8H),4.27(s,2H),3.74(s,3H),3.71–3.62(m,2H) ,3.52(s,2H),3.41–3.34(m,1H),3.05(s,1H),2.95(s,1H),2.41(s,15H),2.31(s,1H),2.26(s,1H ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com