Application of Tryptanthrin Derivatives in Treatment of Plant Virus Diseases
A technology of plant pathogens and derivatives, applied in the fields of applications, plant growth regulators, botany equipment and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
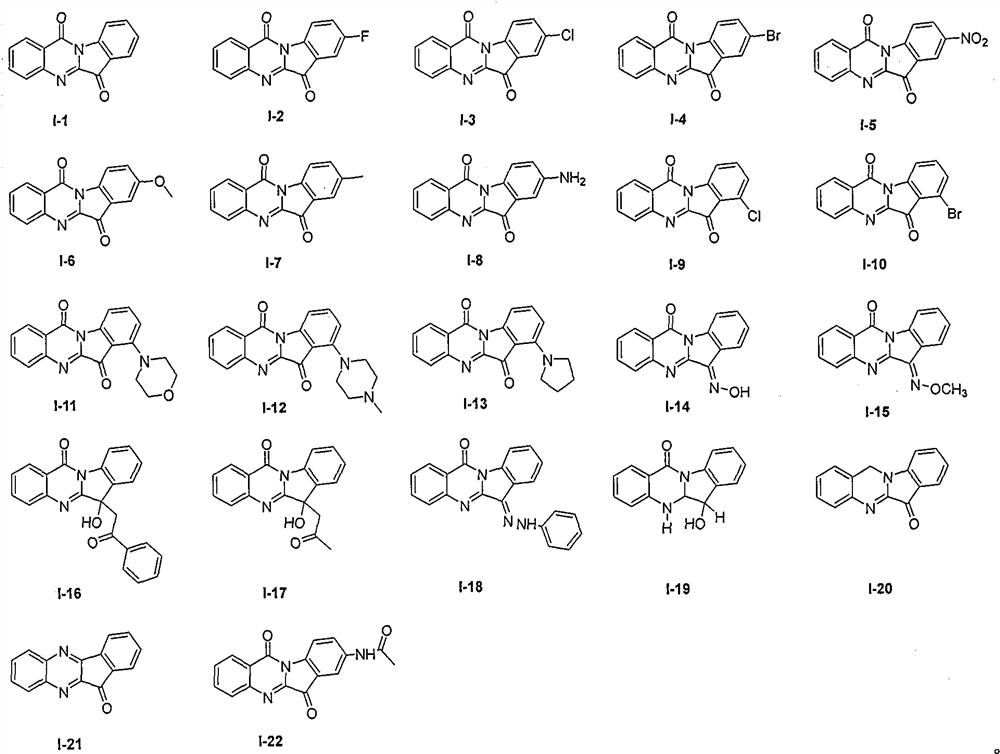
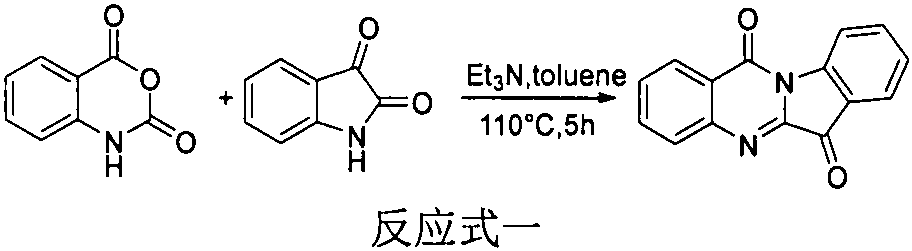
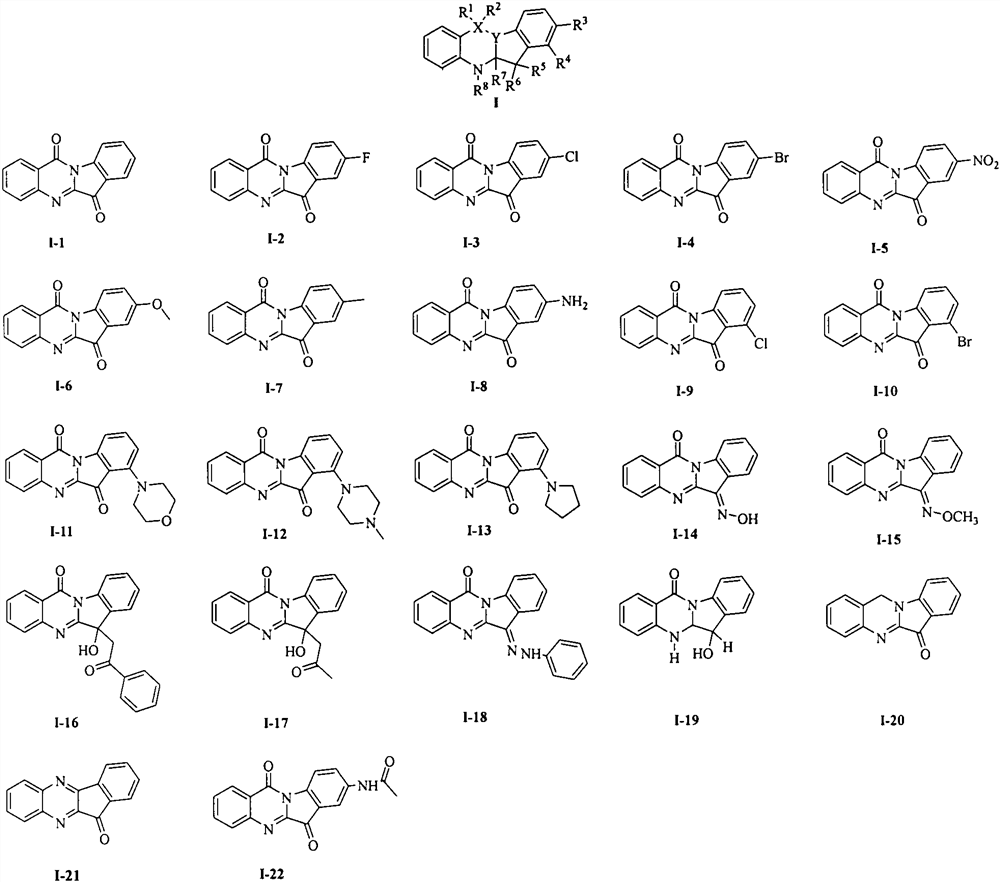
[0011] Embodiment 1: Experimental data of structural formulas of tryptanthrin derivatives I-1~I-22
[0012] I-1: Yellow solid, melting point 267-269°C. 1 H NMR (400MHz, DMSO-d6) δ8.47(d, J=7.9Hz, 1H), 8.31(d, J=7.8Hz, 1H), 7.94(d, J=3.8Hz, 2H), 7.91-7.84 (m, 2H), 7.78-7.69(m, 1H), 7.48(t, J=7.5Hz, 1H). 13 C NMR (100MHz, DMSO-d6) δ182.4, 157.7, 146.4, 145.9, 145.0, 137.7, 135.1, 129.8, 126.9, 124.7, 123.2, 122.8, 117.0.
[0013] I-2: Yellow solid, melting point 282-284°C. 1 H NMR (400MHz, DMSO-d6) δ8.52-8.45 (m, 1H), 8.32 (d, J=7.8Hz, 1H), 7.98-7.92 (m, 2H), 7.82-7.70 (m, 3H).
[0014] I-3: Yellow solid, melting point 296-298°C. 1 H NMR (400MHz, DMSO-d6) δ8.48(d, J=8.4Hz, 1H), 8.34(d, J=7.8Hz, 1H), 8.02-7.88(m, 4H), 7.78-7.72(m, 1H).
[0015] I-4: Brown solid, melting point 260-262°C. 1 H NMR (400MHz, DMSO-d6) δ8.41(d, J=8.4Hz, 1H), 8.33(d, J=7.8Hz, 1H), 8.09-8.04(m, 2H), 7.97(d, J= 3.7Hz, 2H), 7.78-7.73(m, 1H).
[0016] I-5: Brown solid, melting point 268-270°C. 1 H ...
Embodiment 2
[0034] Embodiment 2: the assay of anti-tobacco mosaic virus activity, assay procedure is as follows:
[0035] 1. Virus purification and concentration determination:
[0036] Virus purification and concentration determination were carried out in accordance with the SOP specification for tobacco mosaic virus compiled by the Bioassay Laboratory of the Institute of Elements, Nankai University. After the crude virus extract was centrifuged twice with polyethylene glycol, the concentration was measured and refrigerated at 4°C for later use.
[0037] 2. Compound solution preparation:
[0038] After weighing, the original drug was dissolved in DMF to prepare 1×10 5 μg / mL mother solution, and then diluted with 1‰ Tween 80 aqueous solution to the required concentration; Ningnanmycin preparation was directly diluted with water.
[0039] 3. In vivo protection:
[0040] Select Shanxi tobacco with uniform growth at the 3-5 leaf stage, spray the whole plant, and repeat each treatment 3 t...
Embodiment 3
[0052] Embodiment 3: antibacterial activity test, assay procedure is as follows:
[0053] In vitro bactericidal test, bacterial growth rate determination method (plate method):
[0054] Dissolve a certain amount of medicine in an appropriate amount of acetone, then dilute it with an aqueous solution containing 200 μg / mL emulsifier to the required concentration, then draw 1 mL of the medicine solution into the petri dish, then add 9 mL of medium, shake well and make 50 μg / mL mL of the drug-containing plate, and a plate with 1 mL of sterilized water added as a blank control. Use a puncher with a diameter of 4mm to cut out the bacterial plate along the outer edge of the hyphae, and move it to the drug-containing plate. Each treatment was repeated three times. Place the culture dish in a constant temperature incubator at 24±1°C. After 48 hours, investigate the expanded diameters of the bacteria discs of each treatment, calculate the average value, and compare with the blank con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com