Novel pharmaceutical composition and application thereof
A drug and drug delivery technology, applied in the field of biomedicine, can solve problems such as damage, adverse drug reactions, poor tumor cell selectivity, etc., and achieve the effect of improving the effect of chemotherapy, reducing the occurrence of adverse reactions, and avoiding drug resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
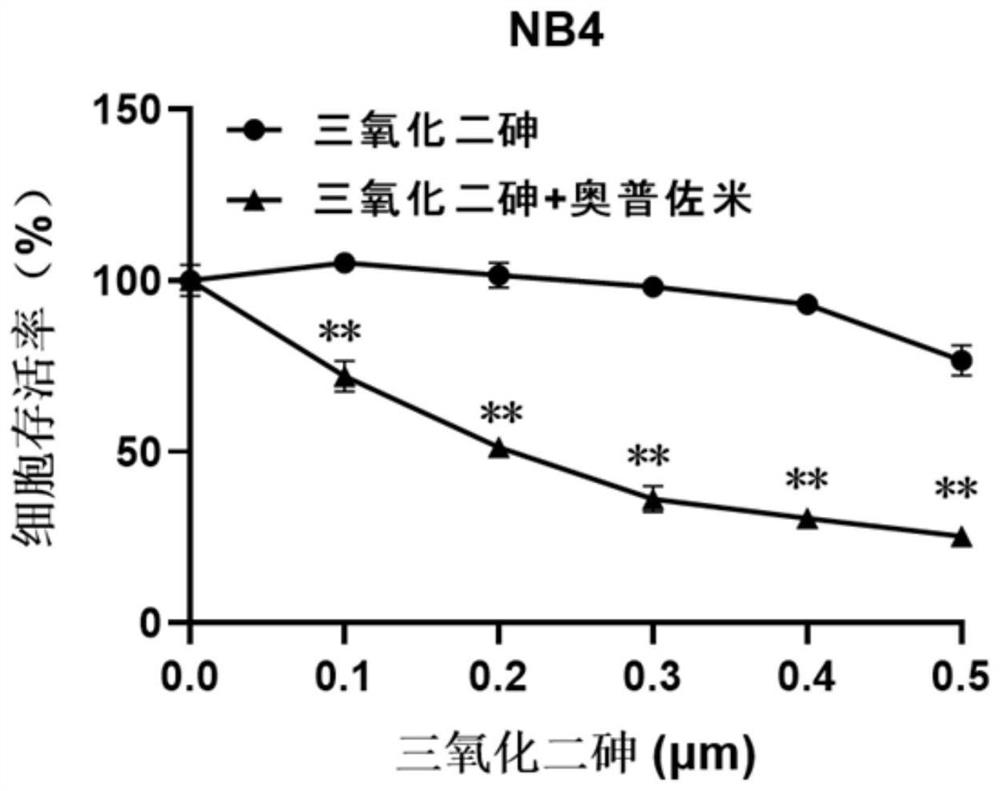
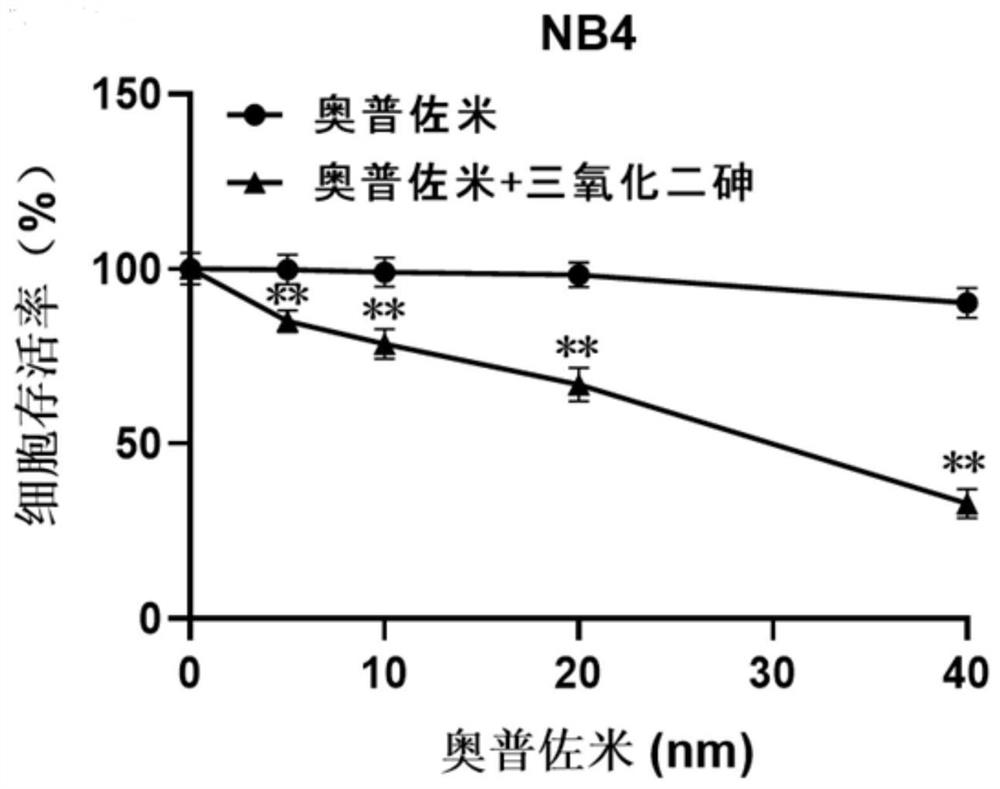
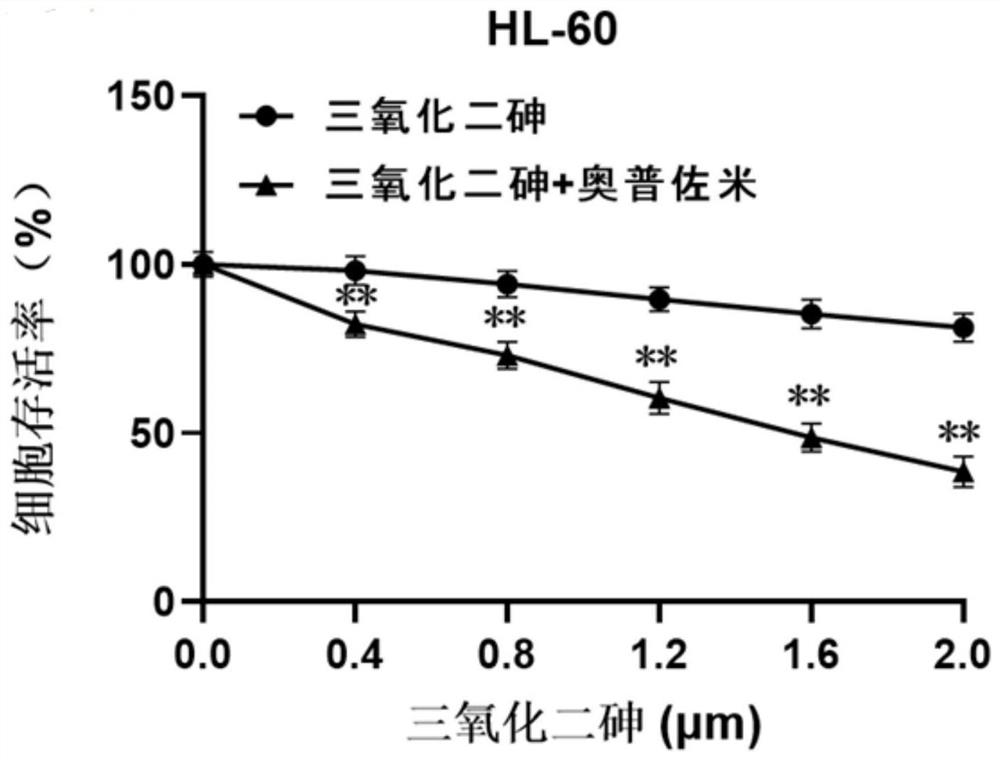
[0031] Experimental Example 1: Cell Experiment
[0032] 1. Cell selection and active culture
[0033] The experimental selection includes acute promyelocytic leukemia cell NB4, acute promyelocytic leukemia cell HL-60; chronic myeloid leukemia cell K562, human liver cancer cell SMMC-7721, and the above-mentioned cells are cultured in RPMI1640+10% FBS complete medium middle. Human colorectal adenocarcinoma cells CaCo-2 were in DMEM+10% FBS complete medium. The above cells were kept at 37°C with 5% CO 2 , cultured in a cell incubator with saturated humidity.
[0034] 2. Experimental method
[0035] After each tumor cell line is in a stable state, spread the cells in a 96-well plate, 10,000 cells per well for acute promyelocytic leukemia cells NB4, acute promyelocytic leukemia cells HL-60, and chronic myelogenous leukemia cells K562; human liver cancer cells SMMC-7721, human colorectal adenocarcinoma CaCo-2, 3000 cells per well. Each well contained 90 μL of complete medium. ...
experiment example 2
[0061] Experimental example 2: mouse APL model experiment (taking acute promyelocytic leukemia cell NB4 as an example)
[0062] 1. Establishment of leukemia orthotopic transplantation mouse model
[0063] The experimental subjects were 5-week-old NOD / SCID mice, which were cultivated in the SPF animal room of the animal experiment center. The feed, litter, and drinking water used by the mice were sterilized at high temperature, and the litter was changed every 2 days. Supplement water and food in time, and feed adaptively for a week. The mice ready for orthotopic transplantation were pretreated 24 hours before the experiment: 1.5eV was irradiated for 7s, and the mice were injected into the tail vein for the orthotopic transplantation experiment the next day.
[0064] 2. Experimental grouping and dosage (5 groups in total)
[0065] Mice were randomly divided into five groups, 6 in each group, negative control group, positive control group, opzomib group, arsenic trioxide group...
Embodiment 1
[0090] In this embodiment, the injection dose has passed the conversion, which is based on the U.S. Food and Drug Administration (FDA) "Estimating the Safe Starting Dose in Clinical Trials for Drug Clinical Trials" (Estimating the SafeStarting Dose in Clinical Trials for Given in Therapeutics in Adult Healthy Volunteers), the injection dose conversion ratio (1:12.3) of the human body and the mouse (ie, the mouse in the experimental verification) required to achieve the same biological effect. The dose in mice was first tested and then the human dose was calculated. According to the dose of mice, opzomib: arsenic trioxide = 0.43mg / kg: 1.53mg / kg, and then according to the injection dose conversion ratio (1:12.3) of human and mouse, 0.035mg: 0.1245mg, of which 0.1245 is 0.083 Mean of -0.166.
[0091] Opzomib combined with arsenic trioxide chemotherapy drug combination, the drug combination consists of opzomib injection and chemotherapy drug arsenic trioxide injection. Refer to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com