Cationic lipid compound, composition containing same and application
A technology of cationic lipids and compounds, applied in medical preparations containing active ingredients, liposome delivery, and medical preparations with non-active ingredients, etc., can solve problems such as high sensitivity and low cell permeability, and achieve rich Kind of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] The synthetic route of compound 1:
[0084]
[0085]
[0086] Step 1: Synthesis of compound 1-1
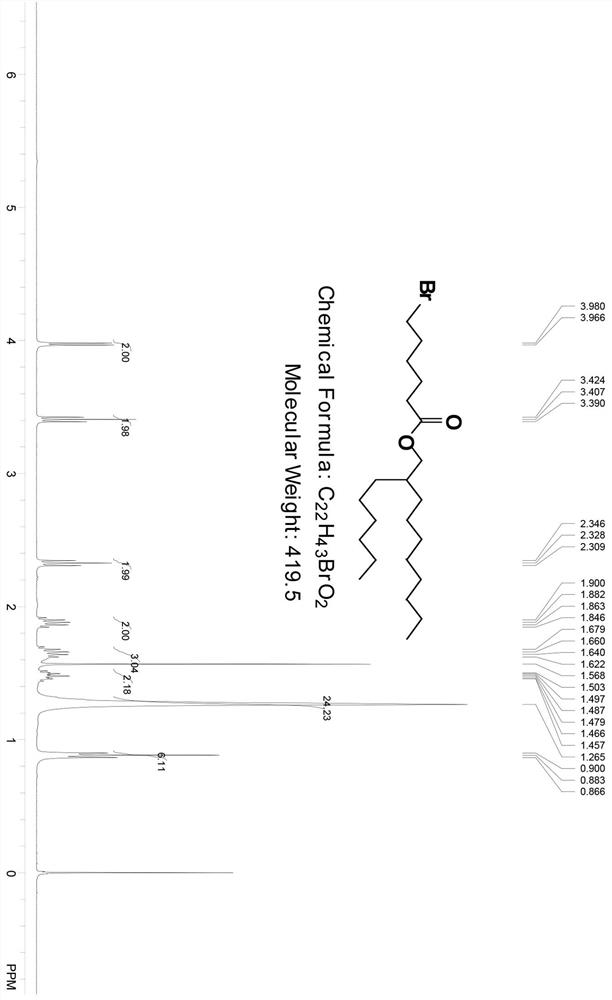
[0087] To 2-hexyldecanol (2.0 g, 8.26 mmol, 1.0 eq) and 6-bromohexanoic acid (1.92 g, 10.0 mmol, 1.2 eq) in dichloromethane (30 mL) was added diisopropylethylamine ( 266.9mg, 2.08mmol, 0.25eq) and DMAP (201.8mg, 1.67mmol, 0.2eq). After the mixture was stirred at room temperature for 5 minutes, EDCI (2.85 g, 14.87 mmol, 1.8 eq) was added and the reaction mixture was stirred at room temperature overnight, then TLC showed complete disappearance of the starting alcohol. The reaction mixture was diluted with DCM (300 mL) and washed with saturated NaHCO 3 (100mL), water (100mL) and brine (100mL) for washing. The combined organic layers were washed with Na 2 SO 4 Drying and removal of the solvent in vacuo afforded the crude product, which was purified by column chromatography (silica gel column, eluent 0-1% EA (volume percent) in n-hexane) and the pure product fractions...
Embodiment 2
[0096] The synthetic route of compound 2:
[0097]
[0098]
[0099] Step 1: Synthesis of compound 2-2
[0100] A mixture of 1,2-epoxytetradecane (500 mg, 2.36 mmol, 1.0 eq) and compound 2-1 (411.1 mg, 3.54 mmol, 1.5 eq) in EtOH (10 mL) was stirred overnight at room temperature. The mixture was concentrated in vacuo, and the residue was purified by silica gel column chromatography (gradient elution, DCM:MeOH=1:0~10:1, volume ratio) to obtain compound 2-2 (720 mg, 92.7%) as a yellow oil. LCMS: Rt: 0.74 min; MS m / z (ESI): 329.3[M+H] + .
[0101] Step 2: Synthesis of compound 2
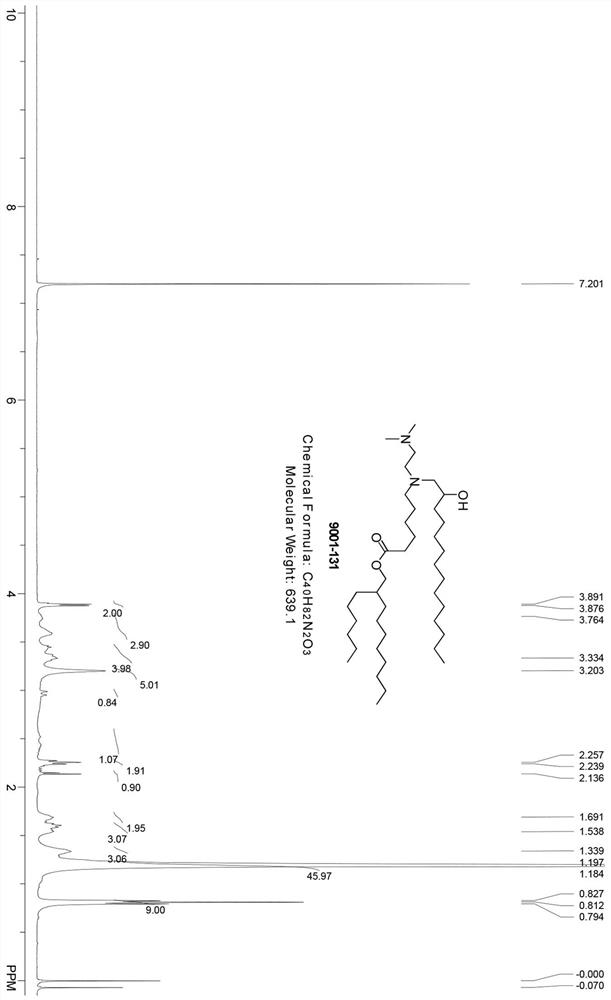
[0102] Contains compound 2-1 (300 mg, 0.91 mmol, 1.0eq), compound 1-1 (460.1 mg, 1.10 mmol, 1.2eq), K 2 CO 3 (377.5 mg, 2.73 mmol, 2.7 eq), Cs 2 CO 3 (80.05 mg, 0.245 mmol, 0.27eq) and a catalytic amount of NaI (36.7 mg, 0.245 mmol, 0.27eq) in acetonitrile (4 mL) were stirred at 80°C overnight. The mixture was filtered and the filtrate was concentrated under vacuum. The residue was purifie...
Embodiment 3
[0106] Synthetic route of compound 3
[0107]
[0108]
[0109] Step 1: Synthesis of Compound 3-2
[0110] A mixture of 1,2-epoxytetradecane (353 mg, 1.67 mmol, 1.0 eq) and compound 3-1 (300 mg, 2.08 mmol, 1.24 eq) in EtOH (10 mL) was stirred overnight at room temperature. The mixture was concentrated in vacuo, and the residue was purified by silica gel column chromatography (gradient elution, DCM:MeOH=1:0~10:1, volume ratio) to obtain compound 3-2 (500 mg, 84.0%) as a yellow oil. LCMS: Rt: 0.730 min; MS m / z (ESI): 357.4[M+H] + .
[0111] Step 2: Synthesis of Compound 3
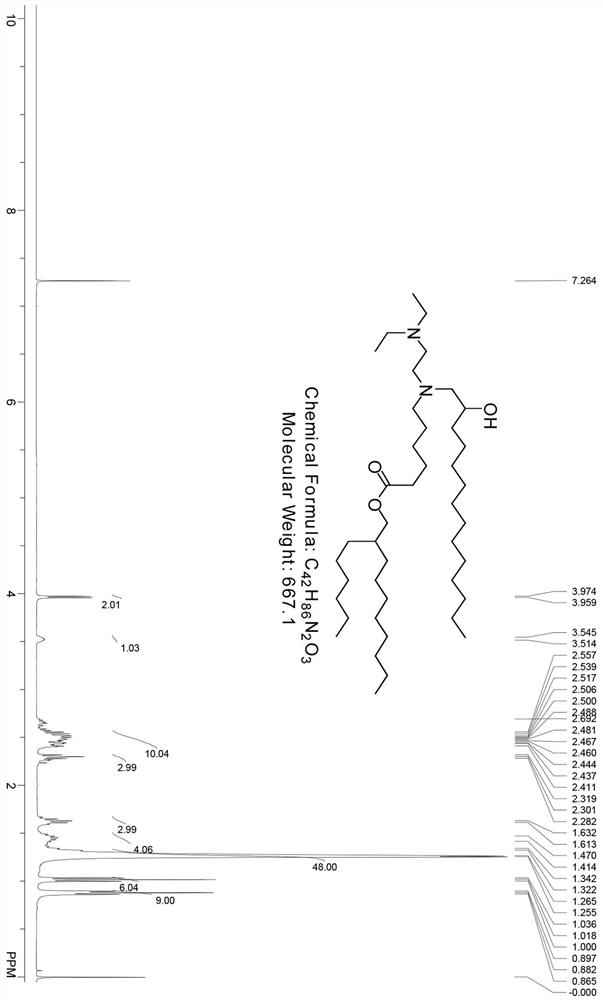
[0112] Contains compound 3-2 (500 mg, 1.4 mmol, 1.0eq), compound 1-1 (704.1 mg, 1.69 mmol, 1.2eq), K 2 CO 3 (483 mg, 3.5 mmol, 2.5 eq), Cs 2 CO 3 (136.8 mg, 0.42 mmol, 0.3 eq) and a catalytic amount of NaI (62.9 mg, 0.42 mmol, 0.3 eq) in acetonitrile (5 mL) were stirred at 80 °C overnight. The mixture was filtered and the filtrate was concentrated under vacuum. The residue was purified by PREP-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com