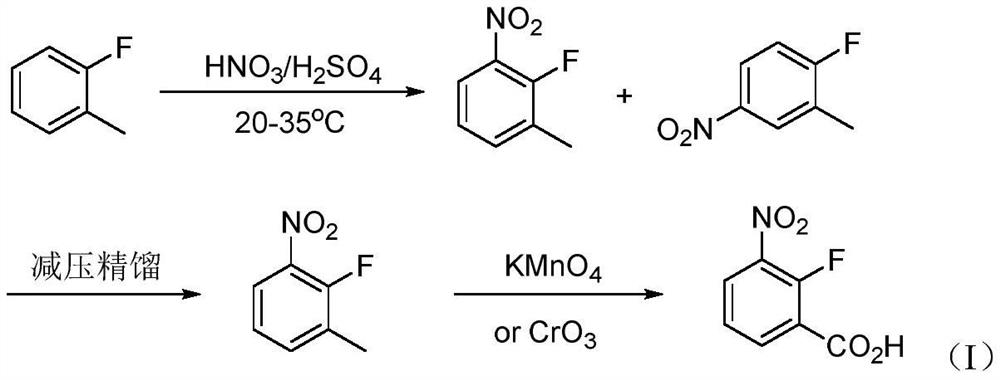
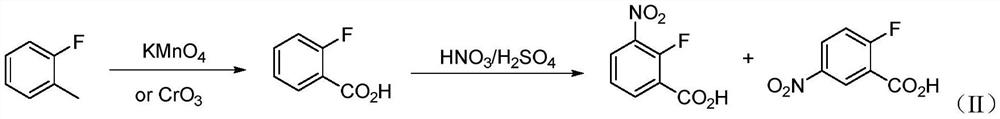
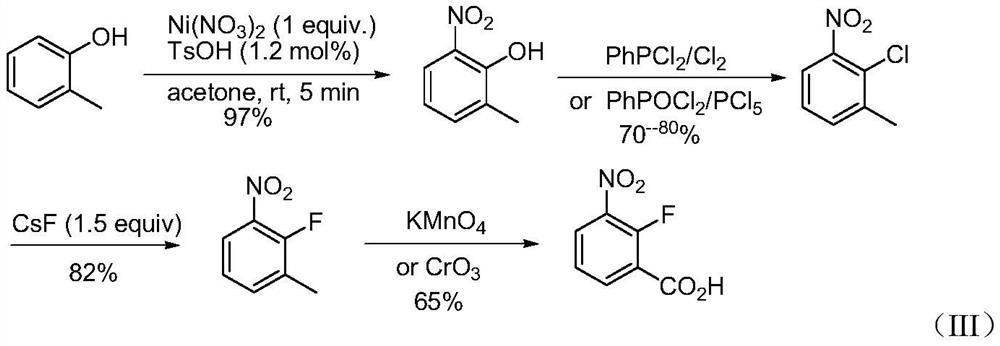
Synthesis method of 2-fluoro-3-nitrobenzoic acid intermediate raw material
A technology of nitrobenzoic acid and a synthesis method, which is applied in the field of synthesis of 2-fluoro-3-nitrobenzoic acid intermediate raw materials, can solve the problems of reduced nitration reaction rate, harsh reaction conditions, unfavorable industrialized production, etc. The effect of raw material cost, improved yield and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1.1 Synthesis of intermediate 2-methyl-6-nitrophenol
[0026]
[0027] Weigh 10.8g o-cresol (100mmol), 18.3g Co(NO 3 ) 2 (100mmol), 0.21g TsOH (1.2mol%) in a 250mL reaction flask, add 100mL acetone (analytical grade, ≥97%), and react under reflux for 8 minutes. After the reaction was completed, it was cooled to room temperature, and the acetone was removed under reduced pressure; simply washed with water to obtain 14.84 g of pale yellow crystals with a yield of 97% and a chromatographic purity of ≥98%. 1 H NMR (400MHz, CDCl 3 ) δ7.83(s, 1H), 7.28(s, 1H), 6.96(s, 1H), 5.0(s, 1H), 2.35(s, 3H).
[0028] 1.2 Synthesis of intermediate 2-chloro-3-nitrotoluene
[0029]
[0030] 14.84g (97mmol) of 2-methyl-6-nitrophenol obtained in step 1, 6.44g C 6 H 5 POCl 2 (33mmol), 66.56g PCl 5 (320 mmol) was all put into a 250 mL round-bottomed flask, slowly heated to 160 ° C, stirred for 5 hours, followed by TLC until the reaction was completed, and the POCl was removed by ...
Embodiment 2
[0039] 1.1 Synthesis of intermediate 2-methyl-6-nitrophenol
[0040]
[0041] Weigh 10.8g o-cresol (100mmol), 18.3g Ni(NO 3 ) 2 (100 mmol) and 0.21 g of TsOH (1.2 mol%) in a 250 mL reaction flask, add 100 mL of acetone (analytical grade, ≥97%), and react under reflux for 5-10 minutes. After the reaction was completed, it was cooled to room temperature, and the acetone was removed under reduced pressure; simply washed with water to obtain 14.84 g of pale yellow crystals with a yield of 97% and a chromatographic purity of ≥98%.
[0042] 1.2 Synthesis of intermediate 2-chloro-3-nitrotoluene
[0043]
[0044] 14.84g (97mmol) of 2-methyl-6-nitrophenol obtained in step 1, 6.44g C 6 H 5 POCl 2 (33mmol), 66.56g PCl 5 (320 mmol) was all put into a 250 mL round-bottomed flask, slowly heated to 160 ° C, stirred for 5 hours, followed by TLC until the reaction was completed, and the POCl was removed by vacuum evaporation 3 . The reaction solution was poured into an ice-water ...
Embodiment 3
[0053] 1.1 Synthesis of intermediate 2-methyl-6-nitrophenol
[0054]
[0055] Weigh 10.8g o-cresol (100mmol), 24.2g Fe(NO 3 ) 3 (100mmol), 0.17g TsOH (1.0mol%) in a 250mL reaction flask, add 100mL acetone (analytical grade, ≥97%), and react under reflux for 5-10 minutes. After the reaction, it was cooled to room temperature, and the acetone was removed under reduced pressure; simply washed with water to obtain 14.68 g of pale yellow crystals with a yield of 96% and a chromatographic purity of ≥98%.
[0056] 1.2 Synthesis of intermediate 2-chloro-3-nitrotoluene
[0057]
[0058] 14.68g (96mmol) of 2-methyl-6-nitrophenol obtained in step 1 and 8.95g C 6 H 5 PCl 2 (50mmol) was added to a 250mL round bottom flask, slowly heated to 160°C, 34.08g Cl 2 (480mmol) was passed into the reaction solution, stirred for 5 hours, followed by TLC until the reaction was completed, and the POCl was removed by vacuum evaporation 3 . The reaction solution was poured into an ice-water...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com