Temperature control supramolecular light capture system and preparation method and application thereof
A light-harvesting and supramolecular technology, which is applied in the preparation of carboxylic acid amides, chemical instruments and methods, and the preparation of organic compounds, can solve the problems of photosynthetic rate decline and affect the light-harvesting mechanism, and achieve high energy transfer efficiency and antenna effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
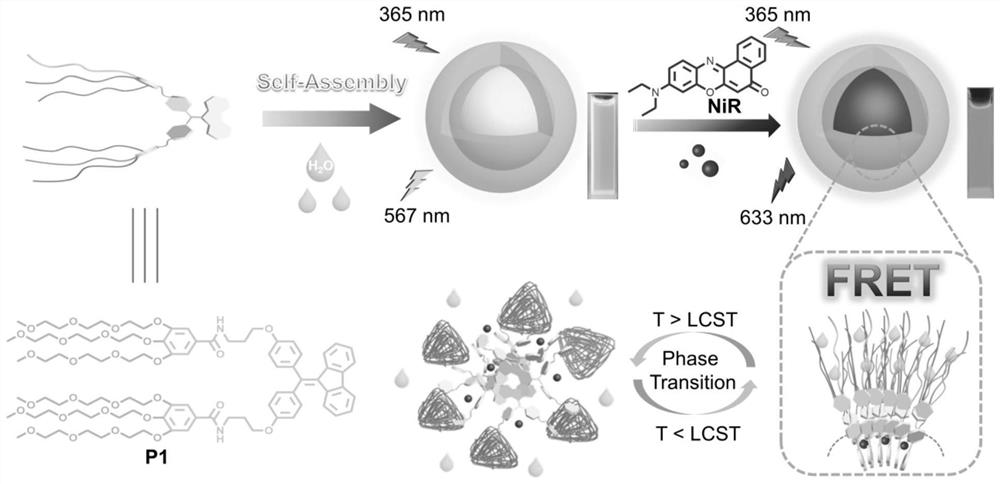
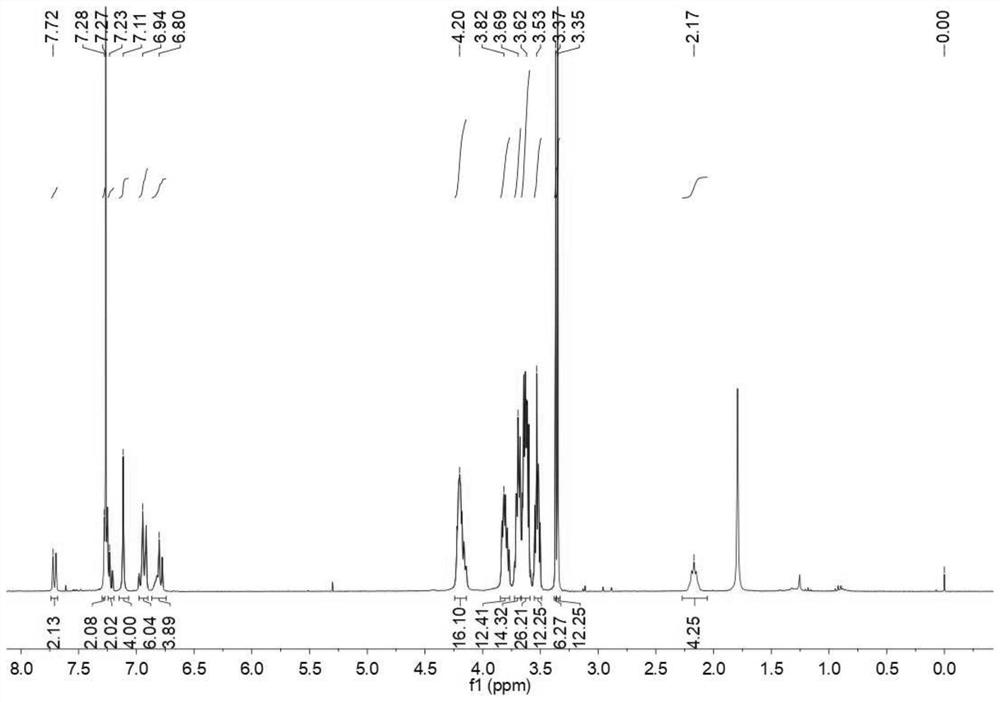
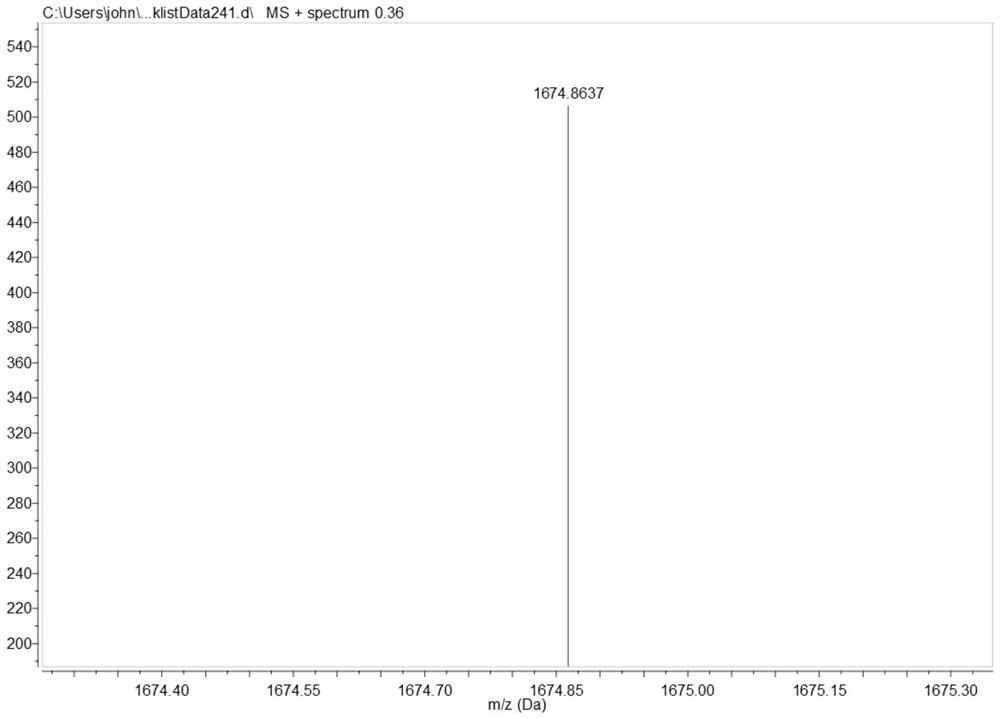
[0043] (1) Synthesis of P1: in N 2 Under protection, add DMAP and the DCM solution of compound A to the flask, ice bath, then add the DCM solution of compound B dropwise, add dropwise Et 3 N, the reaction was stirred overnight at room temperature. After the reaction is complete, stop stirring, add 1M HCl to wash, wash with water, wash with saturated NaCl aqueous solution, combine organic phases, anhydrous MgSO 4 Drying, spin-drying with a rotary evaporator, column chromatography, collecting the spin-dried product to obtain solid powder P1, the yield is 98%. The NMR characterization of compound P1 is shown in the attached figure 2 As shown, the high-resolution mass spectrometry characterization is shown in the attached image 3 shown.
[0044] (2) P1 molecules self-assemble in distilled water driven by hydrophilic-hydrophobic interactions. The critical aggregation concentration of P1 was determined to be 132 μM by light transmittance experiments, and P1 assembled into a sph...
Embodiment 2
[0049] Step (1), step (2) and step (3) are identical with embodiment 1.
[0050] (4) Prepare a 1mM aqueous solution of P1, use ultrasonic method to load NiR, prepare a donor-acceptor solution with D / A=200:1, measure the fluorescence spectrum, and obtain Φ ET 80%, AE=61.
[0051] (5) Prepare a 1 mM aqueous solution of P1, use ultrasonic method to load NiR, prepare a donor-receptor solution with D / A=200:1, and catalyze the C-H alkylation reaction. The conversion and yield are 93% and 90%, respectively. When the temperature of the system was raised to 48°C, the solution became turbid. At this time, the conversion rate and yield dropped to 31% and 4%, respectively. The temperature increase successfully inhibited the catalytic channel, hindered the transformation of light energy into chemical energy, and realized the advanced level of photosynthesis. Bionic.
Embodiment 3
[0053] Step (1), step (2) and step (3) are identical with embodiment 1.
[0054] (4) Prepare the 1mM aqueous solution of P1, use ultrasonic method to load NiR, prepare the donor-acceptor solution of D / A=500:1, measure the fluorescence spectrum, get Φ ET 60%, AE=78.
[0055] (5) Prepare a 1 mM aqueous solution of P1, use ultrasonic method to load NiR, prepare a donor-receptor solution with D / A=500:1, and catalyze the C-H alkylation reaction, and the conversion and yield are 90% and 86%, respectively. When the temperature of the system was raised to 48°C, the solution became turbid. At this time, the conversion rate and yield dropped to 20% and 3%, respectively. The temperature increase successfully inhibited the catalytic channel, hindered the transformation of light energy into chemical energy, and realized the advanced level of photosynthesis. Bionic.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com