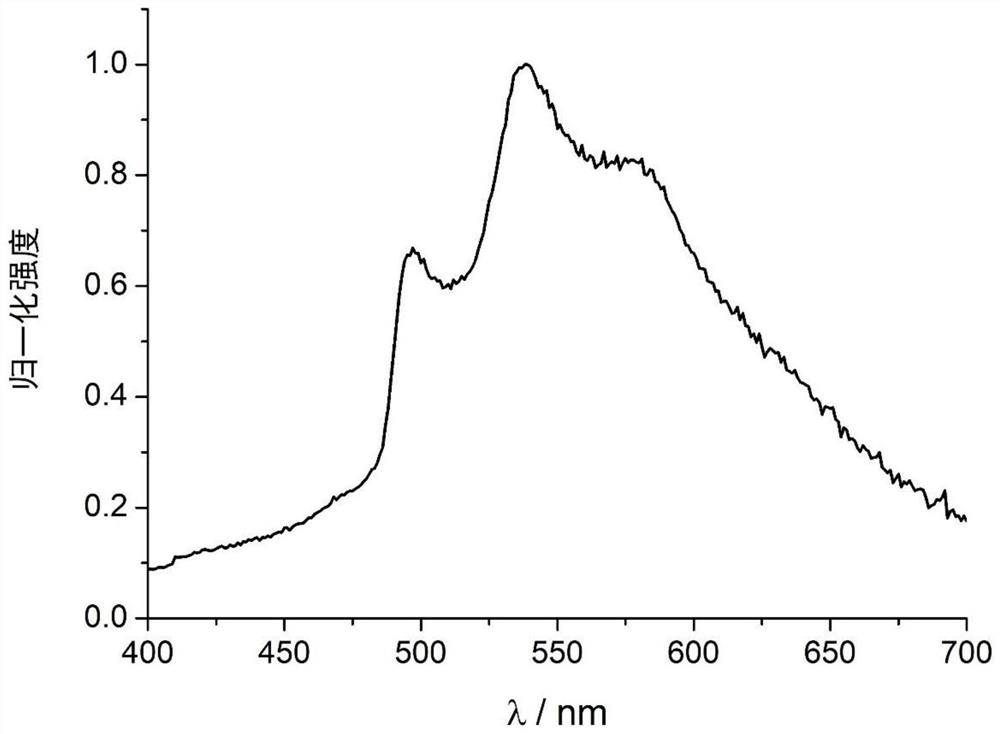
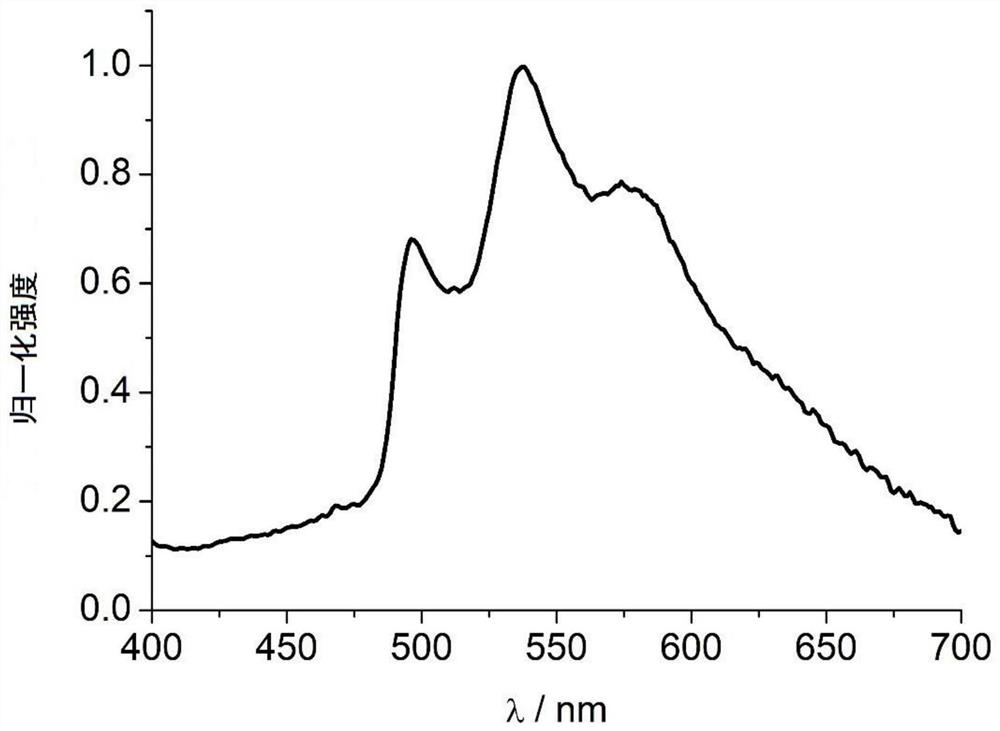
9-acyl-3-iodocarbazole compound and application thereof as phosphorescent material
A compound and carbazole technology, applied in the field of 9-acyl-3-iodocarbazole compounds, can solve the problems of low phosphorescence quantum yield, single structure, large proportion of fluorescence, etc., and achieve adjustable and dispersed luminescence performance Excellent performance and processability, and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Embodiment 1: the preparation of compound II-1
[0100]
[0101] Add 3,6-diiodocarbazole (1g, 2.4mmol) into a round bottom flask, then add 20mL acetic anhydride, and add a little ether boron trifluoride (about 0.02mL) at the same time, react for 1-2 hours, there is a solid product Precipitated, suction filtered, washed and dried to obtain crude 9-acetyl-3,6-diiodocarbazole (compound II-1) with a yield of 90%. The crude product was recrystallized to obtain the pure product as a white solid.
[0102] 1 H-NMR (400MHz, CDCl 3 ):δ8.27(s,2H),7.96(d,J=8.0Hz,2H),7.77(d,J=8.0Hz,2H),2.84(s,3H).
Embodiment 2
[0103] Embodiment 2: the preparation of the preparation of compound II-2
[0104]
[0105] Add polyethylene glycol mercaptan (1g, 0.2mmol) and 9-acetyl-3,6-diiodocarbazole (0.5g, 1mmol) into a round bottom flask, add 10mL dimethylformamide and appropriate amount of potassium carbonate , reacted for 1-2 days under the protection of nitrogen, and the reaction liquid was filtered, precipitated with ether, washed and dried to obtain polyethylene glycol-modified 9-acetyl-3-iodocarbazole (compound II-2).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com