CTLA-4 antibody and preparation method thereof
A technology of antibodies and monoclonal antibodies, applied in botany equipment and methods, biochemical equipment and methods, antibodies, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
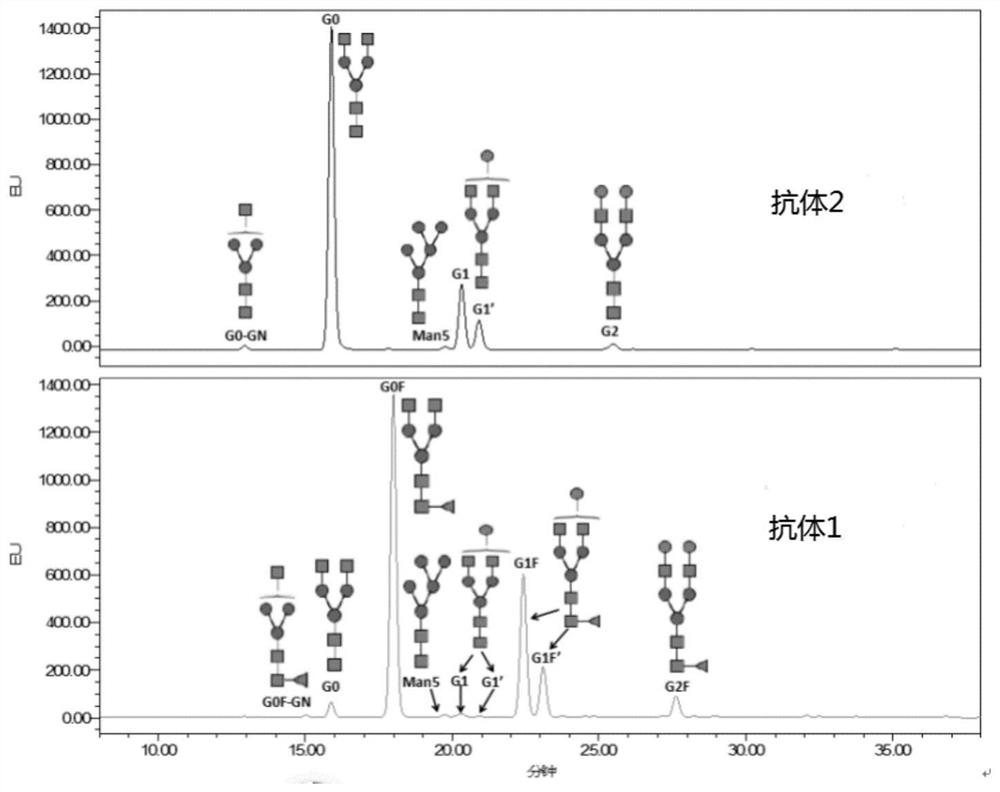
[0084] Example 1. Preparation of Antibody 1 and Antibody 2
[0085] Prepare CHO-BAT (CHO-BAT cells are CHO-K1 cell lines (for example, can come from ATCC CCL-61) Chinese hamster ovary cells adapted to suspension growth through culture, invention patent CN109096399A has disclosed CHO-BAT cells) and CHO-BAT -KF (CHO-BAT-KF cell is a Chinese hamster ovary cell line adapted to suspension growth and knocked out α-(1,6)-fucosyltransferase, which is preserved in the China Center for Type Culture Collection. Number: CCTCC NO: C2017127) cells. The linearized plasmid containing the nucleotide sequence encoding the light chain / heavy chain of the anti-CTLA-4 antibody was transfected into two cell lines by electroporation, and the heavy chain CDR1 of the anti-CTLA-4 antibody was SEQ ID NO: 12. The heavy chain CDR2 is SEQ ID NO: 13, the heavy chain CDR3 is SEQ ID NO: 14, the light chain CDR1 is SEQ ID NO: 15, the light chain CDR2 is SEQ ID NO: 16, and the light chain CDR3 is SEQ ID NO: ...
Embodiment 2
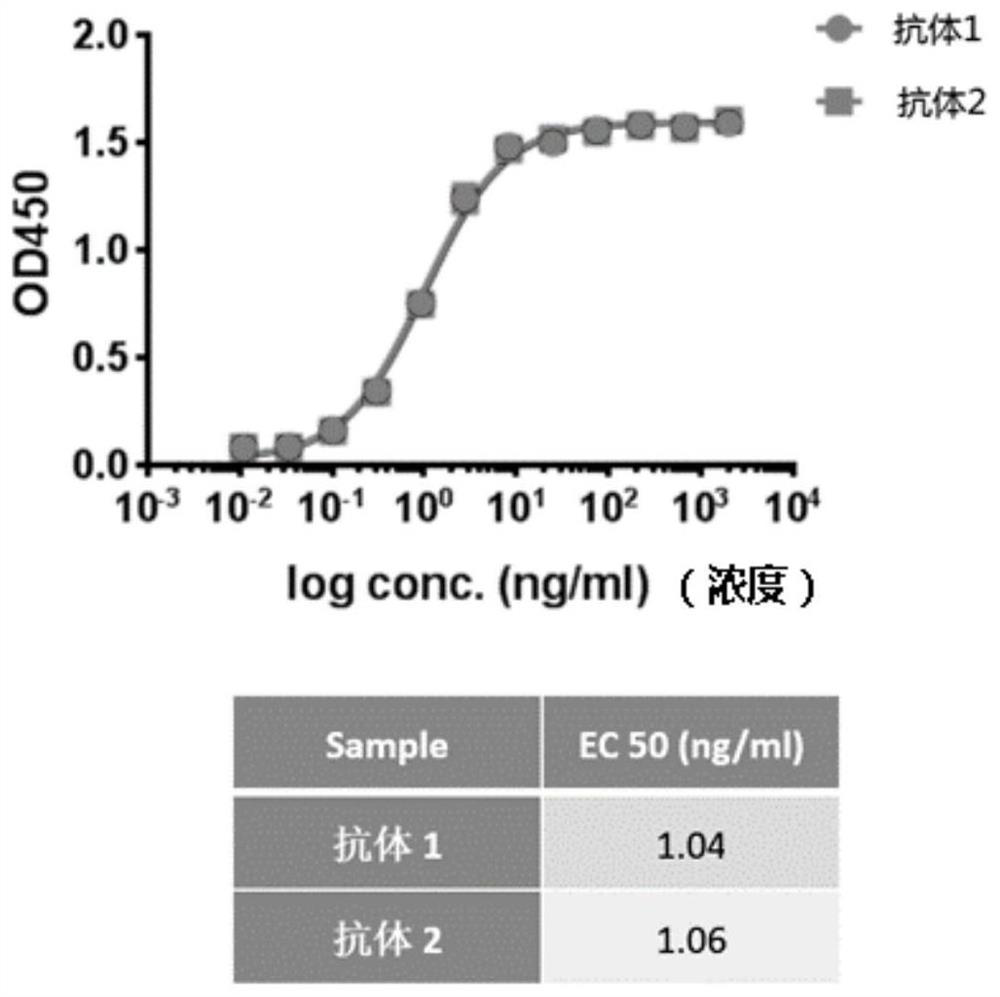
[0091] Example 2. Binding Activity of Antibody 2 Protein and CTLA-4 Antigen in Vitro
[0092] The plasmid used to express the extracellular region of CTLA-4 antigen and the sequence of Fc segment of human IgG1 antibody heavy chain (SEQ ID NO: 6, its amino acid sequence is SEQ ID NO: 5) was transiently transfected into 293F cells.
[0093] 293F cells were cultured with CD 293 TGE Medium (BPM Cell Culture, product number: CM-1156), and when the viability of the cells was above 95%, the cells were passaged to 80-100×10 with fresh medium. 5 Cells / mL, the cell density is about 150-200×10 after 24 hours 5 cells / mL, ready for transfection. Put the plasmid in a water bath at 65°C for 30 minutes, every 100×10 5 1 μg of plasmid was used for cells, and 3 μL of PEI (Polysciences, catalog number: 24765-2) was used for each 1 μg of plasmid. Dilute the plasmid and PEI with culture medium (the sum of the volumes of the two solutions is 5% of the total volume), and let stand at room tempe...
Embodiment 3
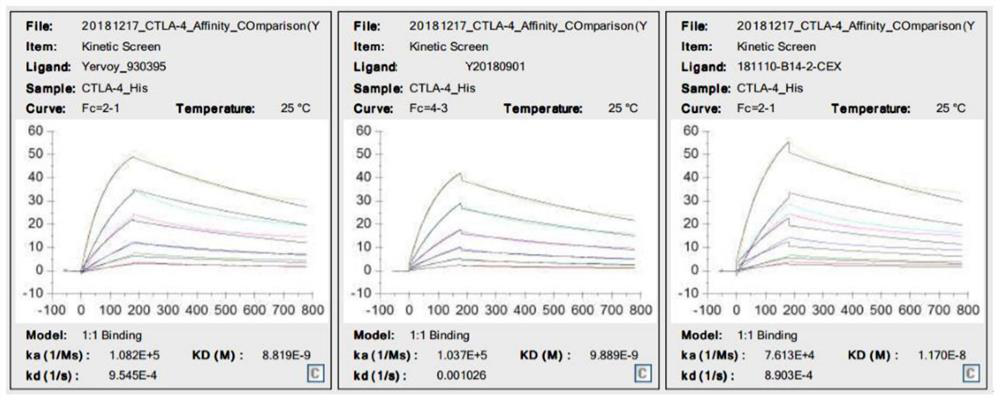
[0100] Example 3. Biological activity of antibody 2 and antibody 1 proteins in vitro
[0101] The schematic diagram of the in vitro biological activity model of anti-CTLA-4 antibody is as follows: Figure 4 .
[0102] Construction of pCMV2-OKT3 (wherein the OKT3 part is the ScFv form of the antibody, its nucleotide sequence is shown in SEQ ID NO: 7, the heavy chain amino acid sequence of OKT3 is shown in SEQ ID NO: 8, and the light chain amino acid sequence is SEQ ID NO: 9) plasmid, the plasmid map is as follows Figure 5 .
[0103] By electroporation, pCMV2-OKT3 was transfected into Raji cells, screened with hygromycin under pressure, mixed with Jurkat-IL2-luciferase cells and stimulated for 6 hours, adding luciferase substrate and using a microplate reader In the method of detecting autofluorescence, positive single clones capable of stimulating Jurkat-IL2-luciferase cells (Promega, JA3005) to produce autofluorescence were screened out and named as Raji-OKT3 cells.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com