Low-saccharification mutant interferon lambda1 as well as expression and purification methods and application thereof
A mutant and interferon technology, applied in the direction of interferon, chemical instruments and methods, biochemical equipment and methods, etc., can solve the problems of increasing immunogenicity and changing glycoproteins, and achieve good uniformity, improved uniformity, Apply promising effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1, the acquisition of low glycation mutant interferon-λ1 gene (IFN-λ1-Nm) of the present invention
[0034]The N-glycosylation site of the IFN-λ1 gene fragment was subjected to site-directed mutation using overlap extension PCR technology, and the codon sequence aac of Asn in the IFN-λ1 gene fragment was replaced with the codon sequence caa of Gln, so that the IFN-λ1 46NWS48 in the amino acid sequence was changed to 46QWS48, and the primer sequence used was: IFNλ1-Nm-F:
[0035] 5’-CAAGCTGAAA TGGAGTTGCA-3' and IFNλ1-Nm-R:5'-TGCAACTCCA TTTCAGCTTG-3', lowercase italics and black bases are codons for Gln.
[0036] pA-αF-IFNλ1 20 The plasmid is used as a template (the construction process of the plasmid is described in detail in the invention patent of the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences, patent No. ZL 200510115720.3), and the first round of PCR uses two pairs of primers to amplify the two target fragments. Increment...
Embodiment 2
[0037] Example 2, Construction of IFN-λ1-Nm Special Expression Vector pA-6IFNλ1-Nm
[0038] Plasmid pUC-IFN-λ1-Nm was digested with restriction endonuclease EcoR I, and an insert fragment of about 818 bp was recovered, ligated with pAO815 plasmid vector (purchased from Invitrogen Company) linearized by EcoR I, transformed into Escherichia coli DH5α, and selected positive Cloning, plasmid extraction, recombinant plasmid pA-IFNλ1-Nm was obtained, the plasmid was verified by restriction enzyme digestion with EcoR I and PstI, and then sequencing verification was carried out. The verification results showed that the insertion position, direction and sequence of the IFN-λ1-Nm gene were obtained ( Consistent with the nucleotide sequence of SEQ ID No.2 in the sequence listing) all correct recombinant methanolophilic yeast expression vectors, the expression vectors contain IFN-λ1-Nm gene expression unit, namely from upstream to downstream including alcohol oxidase (AOX ) promoter, the ...
Embodiment 3
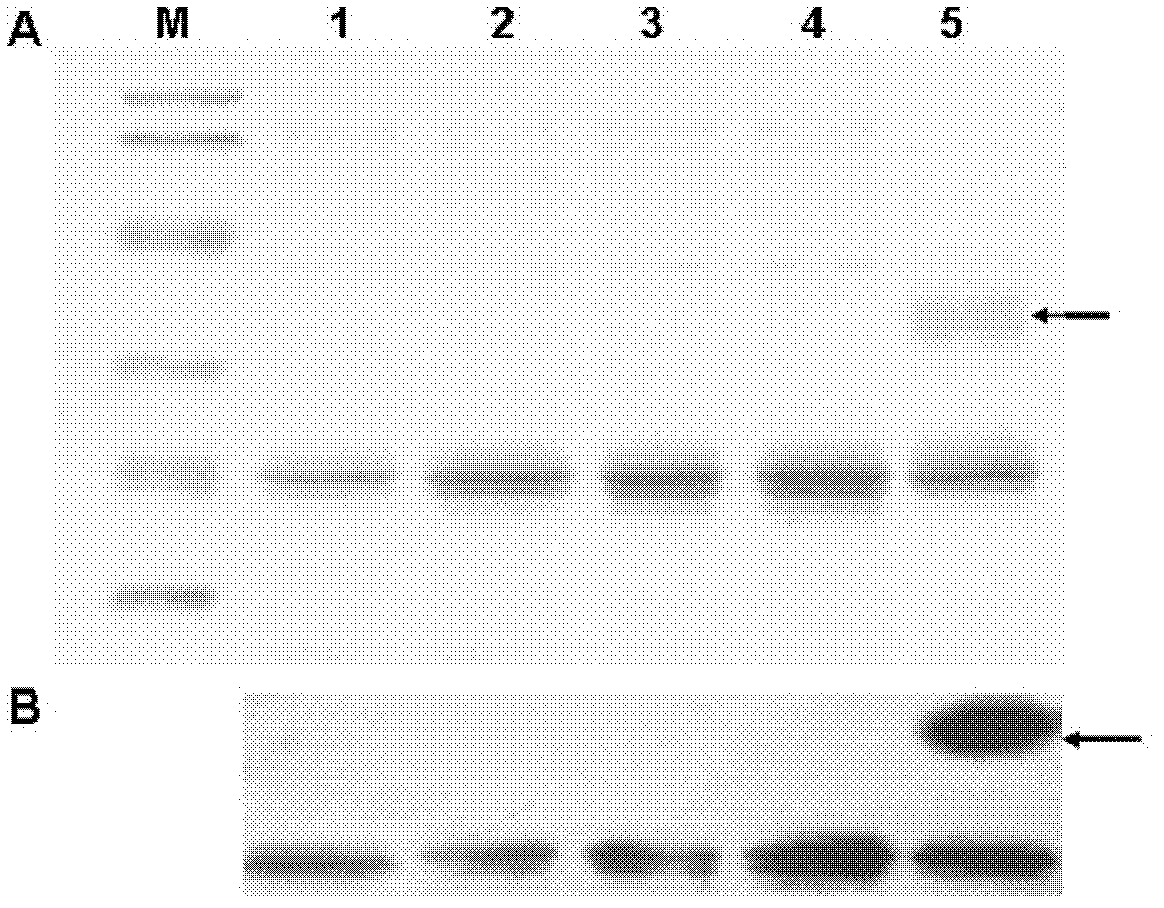
[0040] Example 3. Transformation, expression and purification of IFN-λ1-Nm.
[0041] 1. Transformation of methanolophilic yeast
[0042] Take 10 μg of plasmid pA-6IFNλ1-Nm, digest it with restriction endonuclease Sal I, transform the methanolophilic yeast strain GS115 with the electroporation transformation method, and spread the transformed bacteria on MD medium (1.34% YNB, 4×10 5 % biotin, 2% glucose) at 30°C for 4-6 days, screened to obtain a recombinant yeast strain for expressing the low glycation mutant interferon-λ1 (IFN-λ1-Nm), named GS115 / pA-6IFNλ1- Nm.
[0043] 2. Expression of low glycation mutant interferon-λ1 (IFN-λ1-Nm)
[0044] Inoculate the monoclonal GS115 / pA-6IFNλ1-Nm recombinant yeast strain in 10mL BMG medium (100mM potassium phosphate, pH6.0, 1.34%YNB, 4×10 5 % biotin, 10% glycerol), shake culture at 30°C for 24h, transfer to 100mL BMG medium for 24h, centrifuge to collect the bacteria, resuspend in 500mL BMMY medium (100mM potassium phosphate, pH6.0, 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com