Crystalline nitroxquinoline, and preparation method and application thereof
A nitroxoline and crystallization technology is applied in the field of crystalline nitroxoline and its preparation, and can solve the problems that there is no research and report on nitroxoline crystal forms.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] The preparation example one of the A-type crystal of embodiment 1 nitroxyquinoline
[0101] Weigh 50 mg of nitroquinol, and gradually add methanol (6 mL) at 50° C. until the nitroquinol is completely dissolved into a clear solution. Then, the solution was lowered to room temperature, stirred for 0.5 h, centrifuged (Eppendof Centrifuge 5415, 4000 r / min, 5 min) to separate and collect the wet solid, and dried under reduced pressure at 40 ° C to obtain about 40 mg of yellow long needle-shaped solid, yield: 80%.
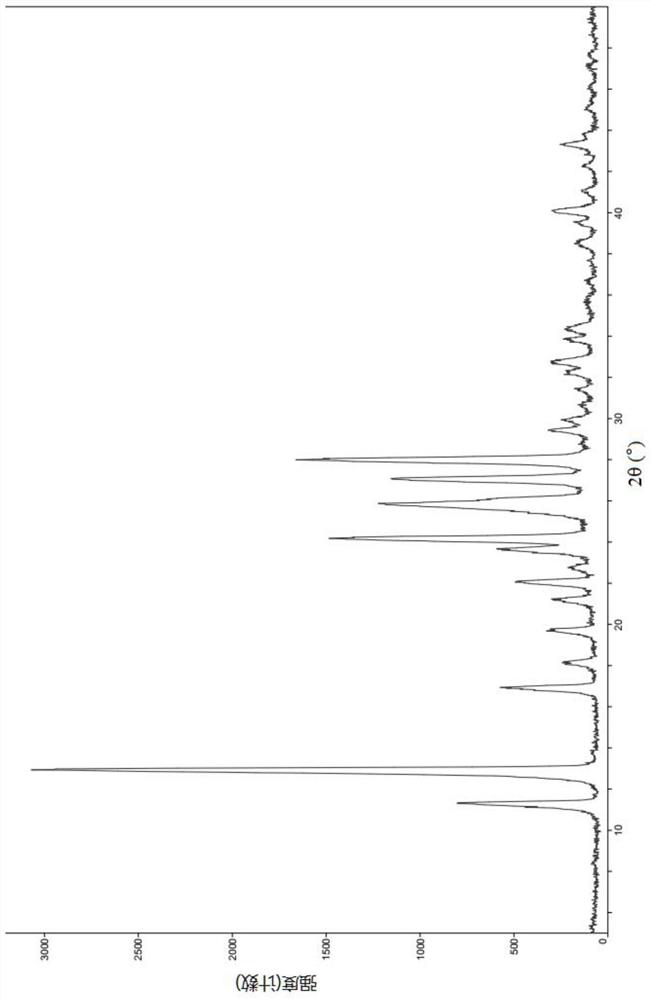
[0102] The X-ray powder diffraction pattern of gained solid sees figure 1 , the X-ray powder diffraction data are shown in Table 1 below, and are defined as type A crystals.
[0103] Table 1 XRPD peak and intensity list of Form A
[0104]
[0105]
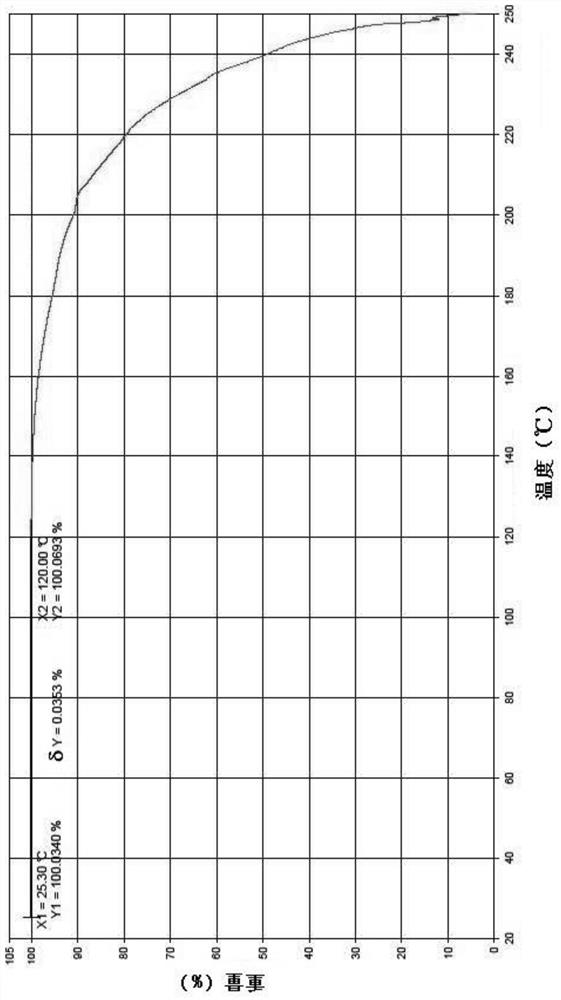
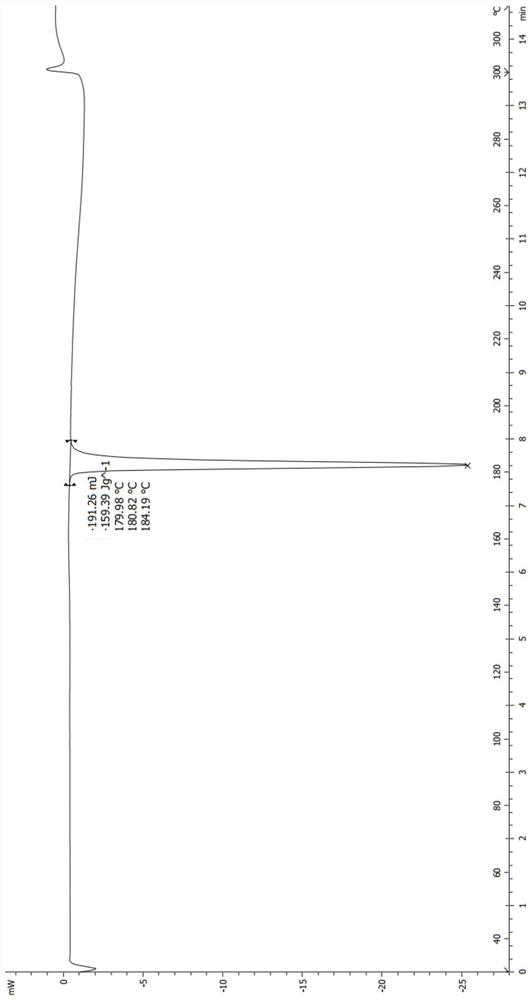
[0106] Weigh about 5 mg of the crystalline sample into a crucible, under nitrogen protection, heat up from 30°C to 250°C at a heating rate of 20°C / min, and hold at 250°C for 1 minute. Its TGA spectrum is show...
Embodiment 2A preparation example 2
[0109]Weigh 50 mg of nitroquinol, and gradually add acetonitrile (2.5 mL) at 60° C. until the nitroquinol is completely dissolved into a clear solution. Then, the solution was lowered to -20°C, stirred for 0.5h, centrifuged (Eppendof Centrifuge 5415, 4000r / min, 5min) to separate and collect the wet solid, and dried under reduced pressure at 40°C to obtain about 35mg of yellow long needle-like solid, which was collected Rate: 70%.
[0110] Its XRPD patterns are researched and compared, and it is confirmed that the product is type A crystal.
Embodiment 3
[0111] The preparation example three of the A-type crystal of embodiment 3 nitroxyquinoline
[0112] Weigh 50 mg of nitroquinol, and gradually add isopropanol (15 mL) at 55° C. until the nitroquinol is completely dissolved into a clear solution. Then, the solution was lowered to -20°C, stirred for 0.5h, centrifuged (Eppendof Centrifuge 5415, 4000r / min, 5min) to separate and collect the wet solid, and dried under reduced pressure at 40°C to obtain about 35mg of a yellow solid, yield: 70 %.
[0113] Its XRPD patterns are researched and compared, and it is confirmed that the product is type A crystal.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com