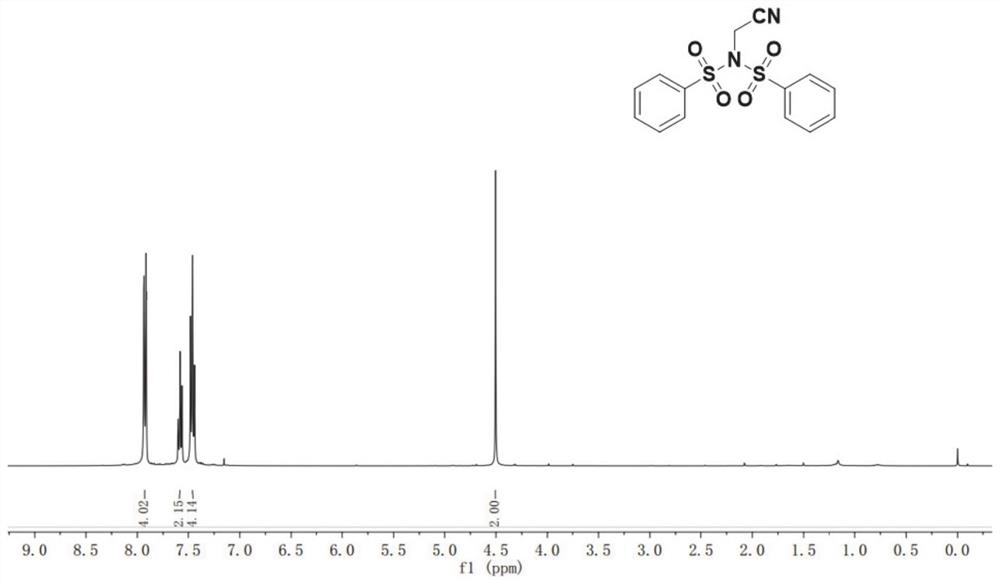
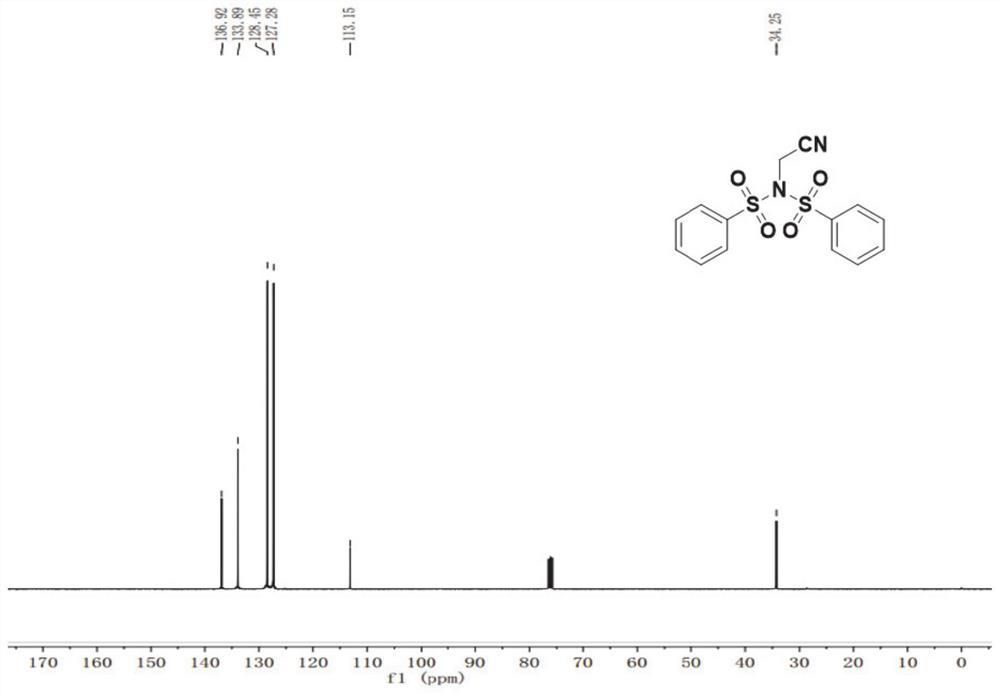
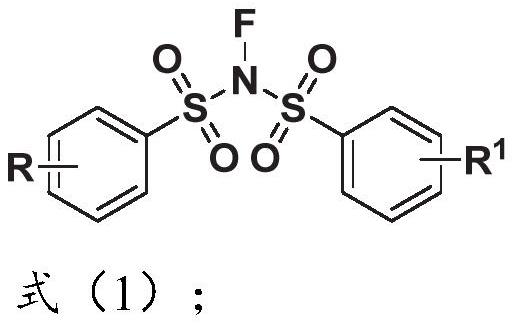
N-acetonitrile dibenzenesulfonimide derivative as well as preparation method and application thereof
A technology based on bisbenzenesulfonimide and acetonitrile, applied in the field of N-acetonitrile bisbenzenesulfonimide derivatives and its preparation, can solve the problems of insufficient product diversity and single type
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Compound 1a (0.2 mmol), compound 1b (0.3 mmol, 1.5 eq) was dissolved in 3 mLDCM, followed by adding CuCl (0.04 mmol, 0.2 eq), PHEN (0.4 mmol, 2 eq), and reacted in an environment of 50 ° C for 16 h. The reaction was monitored by TLC, and the reaction was completed and the reaction was completed. The reaction solution was poured into 30 mL of water, extracted with ethyl acetate (3 × 10 mL), combined with an organic phase, dried over anhydrous sodium sulfate, and then removed the organic solvent by evaporation, and then chromatography (elution) Liquid is v 石油醚 : V 乙酸乙酯 = 20: 1), obtained by obtaining compound 1c, yield of 78%.
Embodiment 2
[0056] Compound 1a (0.2 mmol), compound 1b (0.3 mmol, 1.5 eq) was dissolved in 3 mLDMF, followed by reacting CuCl (0.04 mmol, 0.2 eq), PHEN (0.4 mmol, 2 eq), and reacted in an environment of 50 ° C for 16 h. The reaction was monitored by TLC, and the reaction was completed and the reaction was completed. The reaction solution was poured into 30 mL of water, extracted with ethyl acetate (3 × 10 mL), combined with an organic phase, dried over anhydrous sodium sulfate, and then removed the organic solvent by evaporation, and then chromatography (elution) Liquid is v 石油醚 : V 乙酸乙酯 = 20: 1), obtained from compound 1C, and the yield was 21%.
Embodiment 3
[0058] Compound 1a (0.2 mmol), compound 1b (0.3 mmol, 1.5 eq) was dissolved in 3 mLDCM, then Cui (0.04 mmol, 0.2 eq), PHEN (0.4 mmol, 2 eq), and reacted in an environment of 50 ° C for 16 h. The reaction was monitored by TLC, and the reaction was completed and the reaction was completed. The reaction solution was poured into 30 mL of water, extracted with ethyl acetate (3 × 10 mL), combined with an organic phase, dried over anhydrous sodium sulfate, and then removed the organic solvent by evaporation, and then chromatography (elution) Liquid is v 石油醚 : V 乙酸乙酯 = 20: 1), obtained from compound 1c, yield of 33%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com