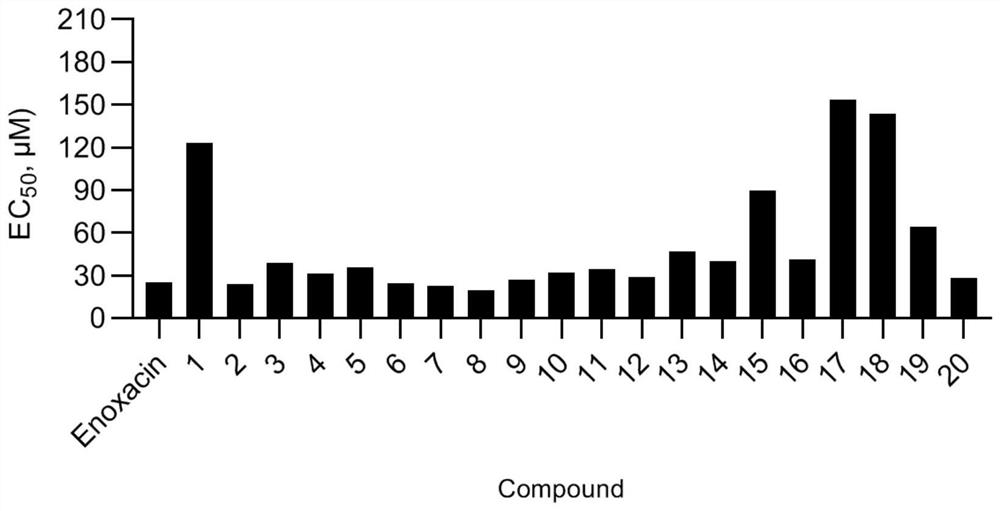
Nitrogen-containing biaromatic ring compounds as well as preparation method and application thereof
A compound and aromatic ring technology, applied in the field of nitrogen-containing biaryl ring compounds, can solve the problems of insufficient inhibitory ability and low affinity of liver cancer cells, and achieve the effects of good anti-cancer effect, strong inhibitory effect and good development prospect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The preparation method of nitrogen-containing biaryl ring derivatives of the present invention can be summarized as follows:
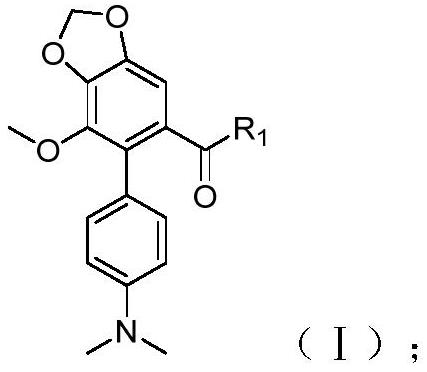
[0042] (a) For compounds of formula (I):
[0043]
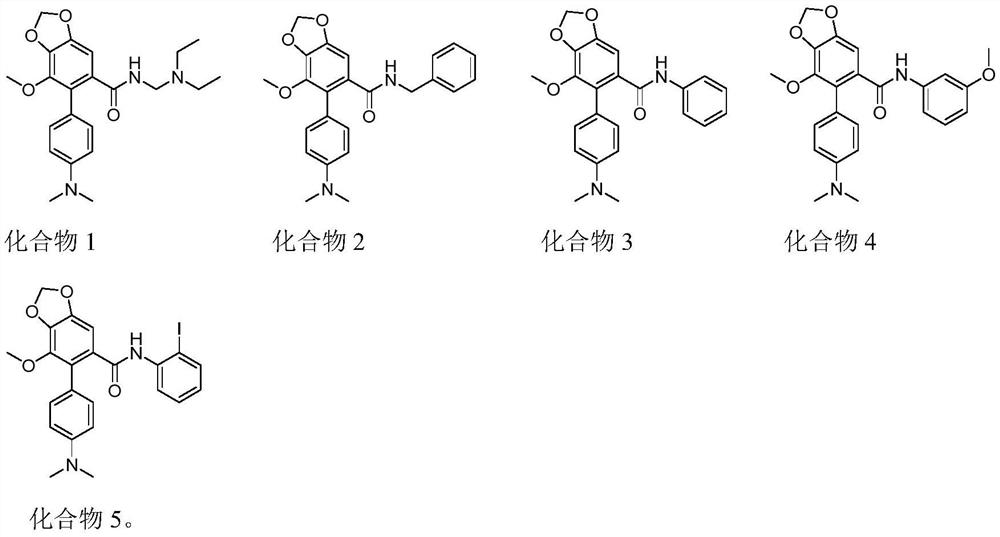
[0044] Take compound A (0.1mmol) in a 25mL round bottom flask, dissolve it with 10mL of dichloromethane, then add SOCl 2 (0.12mmol), reflux at 45°C for 3h, then add the primary amine R 1 H (0.12mmol), Et 3 N (0.12mmol) and pyridine (0.01mmol) were reacted at room temperature for 8h. After the reaction was completed, the reaction solution was poured into water and washed with 3×25mL CH 2 Cl 2After extraction, the combined organic phases were washed with brine 3×25mL, followed by anhydrous Na 2 SO 4 Dry, concentrate and mix the sample with a rotary evaporator, pass through a 100-200 mesh silica gel column to obtain the compound with the structure (I), that is, compound 1-5 of Example 2-6.
[0045] Primary amine R 1 H is a commercially available raw material, purchased from Adamas, Acro...
Embodiment 2
[0057]
[0058] Take the nitrogen-containing biaryl acid obtained in Example 1, that is, compound (A), in a 25mL round-bottomed flask, dissolve it with 10mL of dichloromethane, and then add SOCl 2 (0.12mmol), reflux at 45°C for 3h, then add diethylethylenediamine (CAS: 100-36-7) (0.12mmol), Et 3 N (0.12mmol) and pyridine (0.01mmol) were reacted at room temperature for 8h. After the reaction was completed, the reaction solution was poured into water and washed with 3×25mL CH 2 Cl 2 After extraction, the combined organic phases were washed with brine 3×25mL, followed by anhydrous Na 2 SO 4 Dry, concentrate and mix the sample with a rotary evaporator, pass through a 100-200 mesh silica gel column (PE:EA=4:1) to obtain compound 1 as a yellow solid. 1 H-NMR (300MHz, CDCl 3 ): δ7.18-7.15(2H, d, J=8.7Hz), 6.97(1H, s), 6.74-6.71(2H, d, J=8.7Hz), 6.00(2H, s), 5.60(1H, s), 3.76(3H, s), 3.13-3.10(2H, q, J=5.1Hz), 2.97(6H, s), 2.35-2.28(4H, q, J=7.2Hz), 2.18-2.14(2H , t, J=6.5Hz...
Embodiment 3
[0060]
[0061] Take the nitrogen-containing biaryl acid obtained in Example 1, that is, compound (A), in a 25mL round bottom flask, dissolve it with 10mL of dichloromethane, then add EDCI (0.12mmol) and HOBt (0.12mmol), and then add Benzylamine (CAS: 100-46-9) (0.12mmol) and DMAP (0.01mmol) were reacted at room temperature for 8h. After the reaction was completed, the reaction solution was poured into water and washed with 3×25mL CH 2 Cl 2 After extraction, the combined organic phases were washed with brine 3×25mL, followed by anhydrous Na 2 SO 4 After drying, it was concentrated and mixed with a rotary evaporator, and passed through a 100-200 mesh silica gel column (PE:EA=6:1) to obtain compound 2 as a white solid. 1 H-NMR (300MHz, CDCl 3 ): δ7.18-7.16(3H, m), 7.12(1H, s), 7.09-7.08(1H, m), 6.86-6.85(2H, m), 6.67-6.64(2H, d, J=8.7Hz ), 5.99(2H, s), 5.43(1H, s), 4.24-4.22(2H, d, J=5.1Hz), 3.74(3H, s), 3.97(6H, s). 13 C NMR (75MHz, CDCl 3 ):δ168.43,149.99, 148.29,141...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com