Antibody aiming at chemokine CX3CL1 and application thereof
An antibody and L-CDR3 technology, applied in the field of biomedicine, can solve the problems of loss of biological activity and high probability of immune rejection, and achieve good in vivo safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0147] Example 1 Humanized design of mouse monoclonal antibody 3A5-2 and determination of binding activity of humanized antibody in vitro
[0148] 1.1 Humanized design of mouse monoclonal antibody 3A5-2
[0149] Combining the antibody coding schemes of Kabat and Chothia, the amino acid sequence regions of the six complementarity determinants (CDRs) of the heavy and light chains of the murine antibody 3A5-2 and the framework regions (FRs) supporting the conservative three-dimensional conformation of the antibody were determined. Then search for known human antibody sequences through analysis, select the human antibody heavy chain variable region sequence that is most similar to the mouse antibody, such as IGHV1-69, select its antibody framework region sequence as a template, and convert the mouse antibody heavy chain CDR Combining with human antibody FRs, humanized antibody heavy chain variable region sequences are generated. In the same process, select the IGKV1-13 antibody...
Embodiment 2
[0159] Example 2 Affinity Maturation Engineering of Humanized Antibodies
[0160] 2.1 Design and construction of affinity maturation antibody library
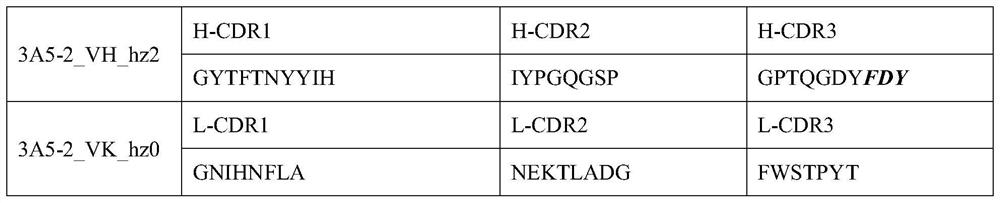
[0161] The humanized antibody 3A5-2_hz20 was selected as a template for affinity maturation engineering. The heavy chain variable region and light chain variable region of 3A5-2_hz20 were connected by GS linker to form a single-chain variable fragment (scFv). The region will remain unchanged in the affinity maturation transformation, and a total of 6 CDRs of the heavy and light chains will be constructed into separate antibody libraries. Based on experience and sequence properties, some amino acids in H-CDR3 are kept unchanged in the modification of affinity maturation, such as figure 2 Invariant amino acids are shown in bold italics.
[0162] Random mutation primers for CDR were designed and synthesized. Only 20% nucleotide mutations were introduced into each mutation position, and there were about 20 base pairs of unmut...
Embodiment 3
[0189] Example 3 Engineering of surrogate antibodies that bind mouse CX3CL1
[0190] 3.1 Construction and screening of engineered antibody library
[0191] The method was as described in the modification of antibody affinity maturation in Example 2. Affinity maturation antibody AM85 was selected as a template for secondary engineering, and an antibody library with random mutations in the full variable region of the heavy and light chains was designed. After 5 rounds of FACS screening with successively decreasing concentrations of mouse CX3CL1 antigen, clones whose FACS binding ability to antigen was stronger than template were obtained, which were re-expanded and cultured, and DNA was extracted and sequenced. Analyze the sequencing results, synthesize new antibody heavy and light chain variable region genes, and construct them into eukaryotic expression vectors. The heavy and light chain expression vectors were cross-combined, transiently transfected into HEK293 cells for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com