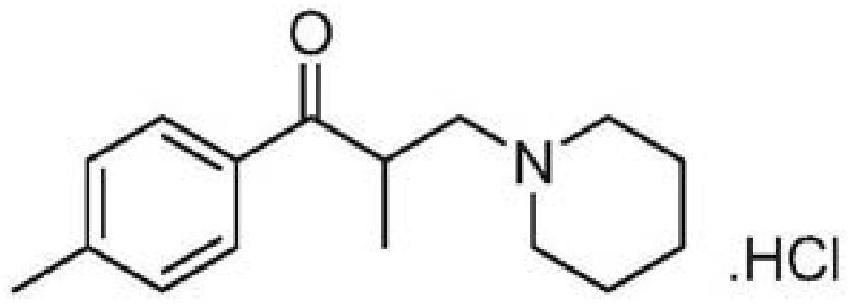
Composition of tolperidone hydrochloride and application thereof
A technology of tolperidone hydrochloride and a composition is applied in the field of the composition of tolperidone hydrochloride, which can solve the problems of not paying attention to the safety of components of tolperidone hydrochloride, achieve good cardiovascular protection, enhance activity, and enhance The effect of immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The present invention also provides a preparation method of the above-mentioned tolperone hydrochloride pharmaceutical composition, comprising the following steps:
[0038] S1, crushing Noni fruit and Schisandra chinensis to obtain a fine powder with a particle size of 30-50 μm;
[0039] S2. Add ethanol aqueous solution to the fine powder material obtained in step S1, and extract in a water bath to obtain an extract; the mass ratio of fine powder material and ethanol aqueous solution described in step S2 is 1:10-15; the mass percentage of the ethanol aqueous solution 70-75%; the conditions of the water-bath extraction are: 2-3 hours of water-bath extraction at 70-85°C;
[0040] S3. Add tolperone hydrochloride and cyclodextrin to the extract obtained in step S2, mix well, concentrate under reduced pressure, dry and pulverize to obtain the pharmaceutical composition of tolperone hydrochloride; the cyclodextrin The added amount of the essence is 0.03-0.05% of the mass of ...
Embodiment 1-6
[0044] Embodiment 1-6 a kind of tolperone hydrochloride pharmaceutical composition and preparation method thereof
[0045] The raw materials and consumption (parts by weight) of the tolperone hydrochloride pharmaceutical composition described in Examples 1-6 are shown in Table 1.
[0046] Table 1
[0047] Example 1 Example 2 Example 3 Example 4 Example 5 Example 6 Tolperone Hydrochloride / part 20 50 25 45 40 35 Noni fruit / serve 10 25 11 24 20 15 Schisandra / serving 16 8 14 9 12 10
[0048] The preparation method of above-mentioned tolperone hydrochloride pharmaceutical composition, comprises the following steps:
[0049] S1, pulverizing noni fruit and schisandra to obtain a fine powder with a particle size of 40 μm;
[0050] S2, add ethanol aqueous solution to the fine powder material that step S1 obtains, extract in water bath, obtain extract; The mass ratio of fine powder material described in step S2 and ethanol aq...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com