Preparation method of 2-bromo-5-fluoro-4-nitroaniline
A technology of nitroaniline and nitric acid, applied in the field of chemistry, can solve the problems of poor safety, high toxicity, and high production process cost, and achieve the effects of low cost, simple and easy-to-obtain raw materials, and high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
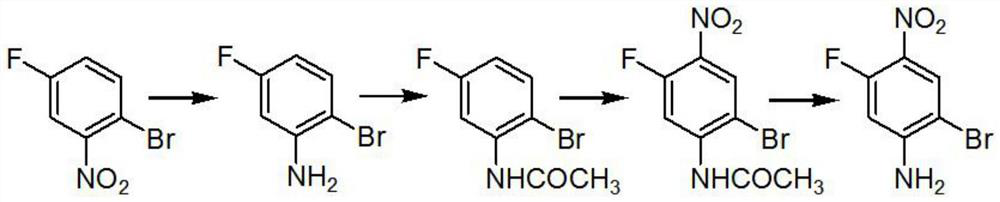
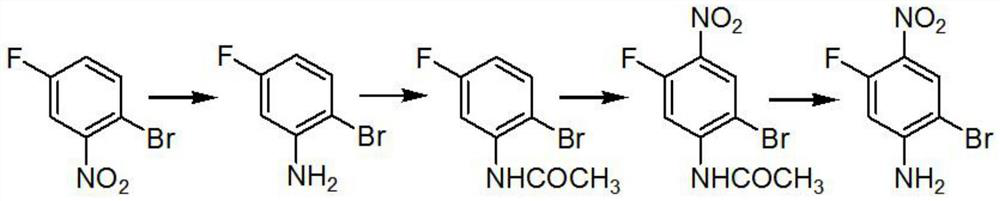
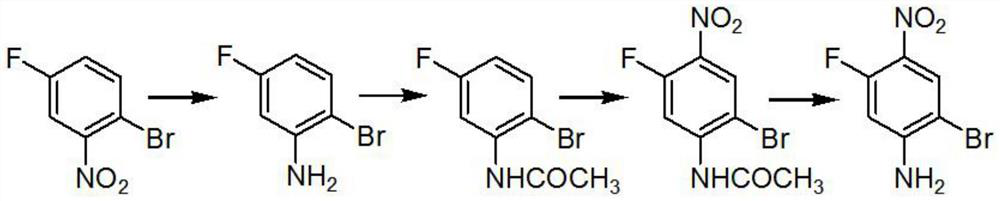
[0017] A preparation method of 2-bromo-5-fluoro-4-nitroaniline, characterized in that: the reaction chemical formula is as follows
[0018]
[0019] The preparation method is as follows:
[0020] 1. A preparation method of 2-bromo-5-fluoro-4-nitroaniline, characterized in that: the reaction chemical formula is as follows
[0021]
[0022] The preparation method is as follows: the consumption is calculated according to the ratio of parts by weight,
[0023] Step A: Add 1.3 kg of iron powder and 0.1 kg of ammonium chloride to the reaction system, add 1 kg of raw materials under reflux, and adjust the base after the reaction is completed. The base is 30% NaOH, adjusted to PH=9, and the product is obtained by steam distillation for use;
[0024] Step B: Add 0.6 kg of 99% acetic anhydride to 1 kg of the product of step A, raise the temperature to 80° C. and keep for 1 hour, and the reaction product is ready for use;
[0025] Step C: add 6.5kg of the product in step B to 6.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com