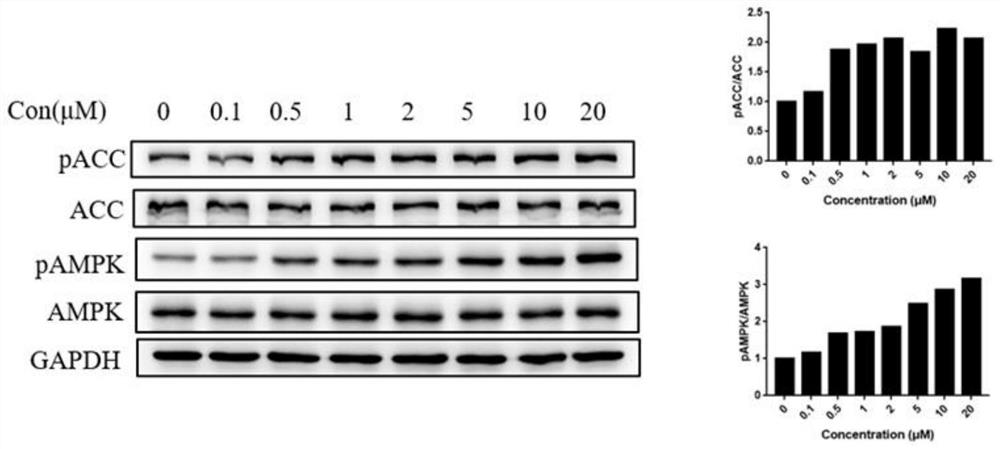
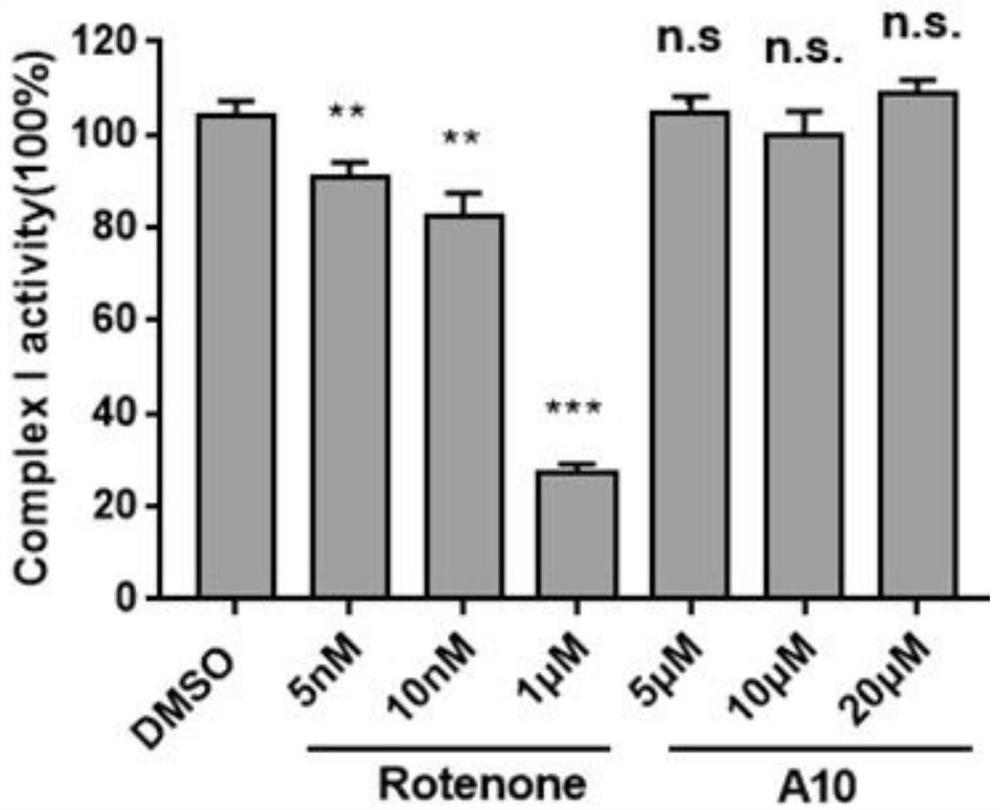
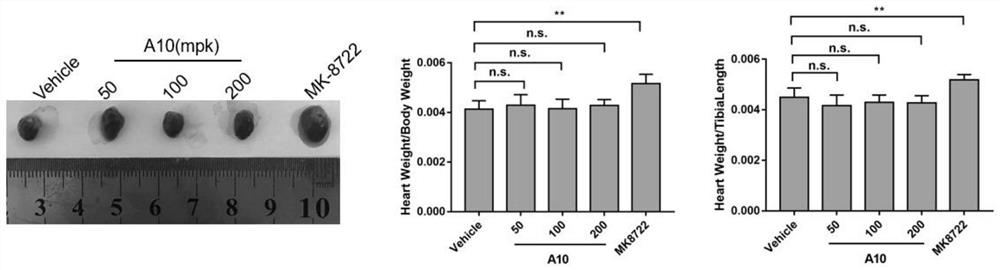
AMPK agonist compound or pharmaceutically acceptable salt or ester or solvate thereof and application thereof
A solvate, compound technology, applied in the field of AMPK agonist compounds, salts or esters or solvates, to achieve the effect of potent AMPK agonist activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] 2-(4-Chlorophenoxy)-N-(4-(4-fluorobenzyl)oxy)phenyl)-2-methylpropanamide (Compound A-1)
[0056]
[0057] Dissolve p-aminophenol (3.27g, 30mmol), imidazole (2.7g, 45mmol) in DCM (50mL), slowly add tert-butyldimethylsilyl chloride (TBSCl) (5.88g, 39mmol) under stirring at room temperature, and react at room temperature 1h. The reaction solution was filtered with suction, and the filtrate was concentrated to obtain a pale yellow oily liquid, compound I-2.
[0058] Add oxalyl chloride (5.1mL, 60mmol) dropwise to a solution of 2-(4-chlorophenoxy)-isobutyric acid (6.44g, 30mmol) in dichloromethane (30mL) under ice-bath conditions, add DMF (2drops) , react at room temperature for 3 hours. The organic solvent was distilled off under reduced pressure to obtain a residue, which was diluted with dichloromethane (15 mL) to obtain a freshly prepared acid chloride solution in DCM. It was added to compound I-2, TEA (6.3 mL, 45 mmol) in dichloromethane (25 mL) under ice-cooling,...
Embodiment 2
[0062] 2-(4-Chlorophenoxy)-N-(4-(4-methoxybenzyl)oxy)phenyl)-2-methylpropanamide (Compound A-2)
[0063]
[0064] With reference to the method of Example 1, 4-fluorobenzyl bromide was replaced by 4-methoxybenzyl chloride to obtain compound A-2: 1 H NMR (300MHz, DMSO-d 6 )δppm 9.84(s, 1H), 7.53(d, J=8.8Hz, 2H), 7.34(dd, J=8.3, 5.2Hz, 4H), 7.00-6.86(m, 6H), 4.98(s, 2H) , 3.75(s, 3H), 1.52(s, 6H). 13 C NMR (75MHz, DMSO-d 6 )δppm 171.70, 154.52, 153.64, 133.36, 131.75, 129.82, 129.71, 129.09, 126.13, 122.00, 121.34, 115.27, 114.99, 114.65, 80.85, 68.59, 24.72 / m - .
Embodiment 3
[0066] N-(4-((4-chlorobenzyl)oxy)phenyl)-2-(4-chlorophenoxy)-2-methylpropanamide (Compound A-3)
[0067]
[0068] With reference to the method of Example 1, 4-fluorobromobenzyl was replaced by 4-chlorobromobenzyl to obtain compound A-3: 1 H NMR (300MHz, DMSO-d 6 )δppm 9.86 (s, 1H), 7.54 (d, J = 8.9Hz, 2H), 7.45 (brs, 4H), 7.34 (d, J = 8.8Hz, 2H), 6.94 (dd, J = 8.9, 2.8Hz , 4H), 5.07(s, 2H), 1.52(s, 6H). ESI-MS: m / z 452.1 [M+Na] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com