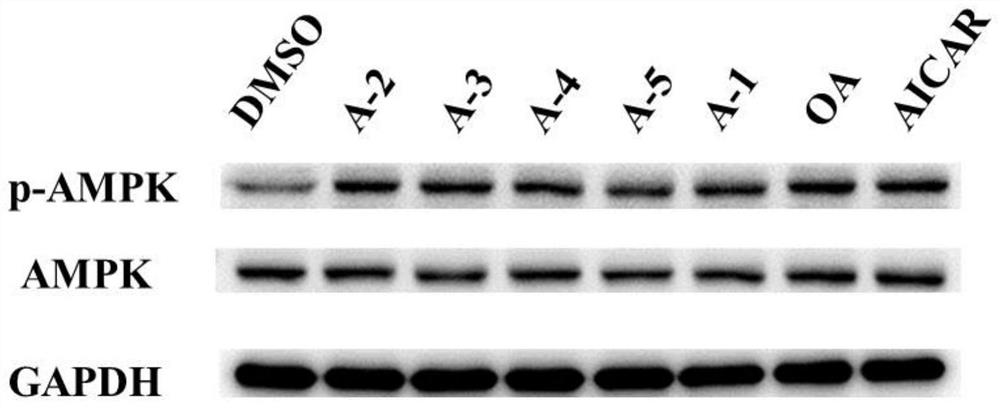
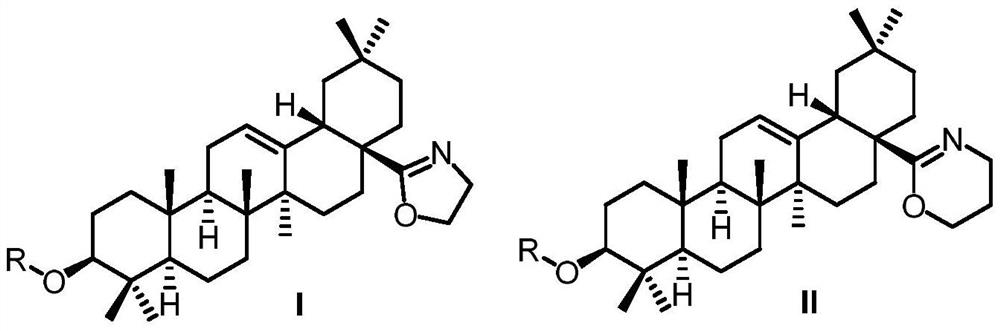
Derivatives of oleanolic acid and their medicinal uses
A technology of oleanolic acid and derivatives is applied in the field of biomedicine to achieve the effects of strong AMPK agonistic activity, good oral bioavailability and good safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] 12-ene-3β-acetoxy-28-(oxazolin-2-yl)-oleanane (Compound A-2)
[0048]
[0049] Dissolve oleanolic acid (OA, 10g, 21.9mmol) in pyridine (150mL), add 4-dimethylaminopyridine (0.26g, 2.19mmol), slowly add acetic anhydride (8.3mL, 87.6mmol), and stir at room temperature overnight. After the reaction is complete, add 1N hydrochloric acid (300mL), extract with ethyl acetate (300mL×3), wash with saturated brine (300mL×3), dry over anhydrous sodium sulfate, concentrate under reduced pressure, make a slurry (petroleum ether: dichloromethane =50:1) Suction filtration to obtain compound I-1 (white solid, 8.2 g, yield 75%).
[0050] Dissolve compound I-1 (5 g, 10 mmol) in anhydrous dichloromethane (80 mL), slowly add oxalyl chloride (1.7 mL, 20 mmol) and N,N-dimethylformamide (5 drops) dropwise under stirring, React at room temperature for 5 hours. After the reaction was detected by TLC, the solvent was evaporated under reduced pressure to obtain compound I-2 (yellow solid, 5...
Embodiment 2
[0054] Oleanane-12-ene-28-(oxazolin-2-yl)-3β-ol
[0055]
[0056] Compound A-2 (3.5g, 6.6mmol) was dissolved in 50mL of methanol, potassium hydroxide (3.7g, 66mmol) was added, heated to 50°C and stirred to react, TLC detected that the reaction was complete. After the reaction was completed, cool to room temperature, add 50 mL of water, extract with ethyl acetate (50 mL×3), wash with saturated brine (50 mL×3), dry over anhydrous sodium sulfate, concentrate under reduced pressure, and perform silica gel column chromatography (petroleum Ether: ethyl acetate = 10: 1) purification to obtain compound A-1 (white solid, 3.0 g, yield 95%): 1 H NMR(300MHz,DMSO)δ5.36-5.18(m,1H),4.26-4.03(m,2H),3.88-3.63(m,2H),3.27-3.15(m,1H),2.93-2.77(m ,1H),1.13(s,3H),0.99(s,3H),0.94(s,3H),0.90(s,6H),0.78(s,3H),0.76(s,3H).ESI-MS: m / z 482.4[M+H] + .
Embodiment 3
[0058] 12-en-3β-propionyloxy-28-(oxazolin-2-yl)-oleanane (Compound A-3)
[0059]
[0060] Dissolve compound A-1 (200mg, 0.4mmol) in pyridine (3mL), add 4-dimethylaminopyridine (5mg, 0.04mmol) and propionic anhydride (133uL, 1mmol) successively, stir at room temperature and react, and TLC detects that the reaction is complete After that, 1N hydrochloric acid (5 mL) was added, extracted with ethyl acetate (5 mL×3), washed with saturated brine (5 mL×3), dried over anhydrous sodium sulfate, concentrated under reduced pressure, and subjected to silica gel column chromatography (petroleum ether: acetic acid Ethyl ester=10:1), to obtain compound A-3 (white solid, 178mg, yield 80%): 1 H NMR (300MHz, CDCl 3 )δ5.32-5.20(m,1H),4.58-4.42(m,1H),4.22-4.03(m,2H),3.91-3.71(m,2H),2.94-2.79(m,1H),2.32( q,J=7.6Hz,2H),0.93(s,6H),0.90(s,3H),0.86(s,9H),0.76(s,3H).ESI-MS:m / z 538.5[M+H ] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com