A kind of synthesis technique of favipiravir intermediate 3,6-dichloro-2-cyanopyrazine
A synthetic process, favipiravir technology, applied in the field of synthetic process of favipiravir intermediate 3,6-dichloro-2-cyanopyrazine, can solve low yield, reduce production cost, synthetic route Long steps and other problems, to achieve the effect of reducing energy consumption, low cost, and shortening the production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
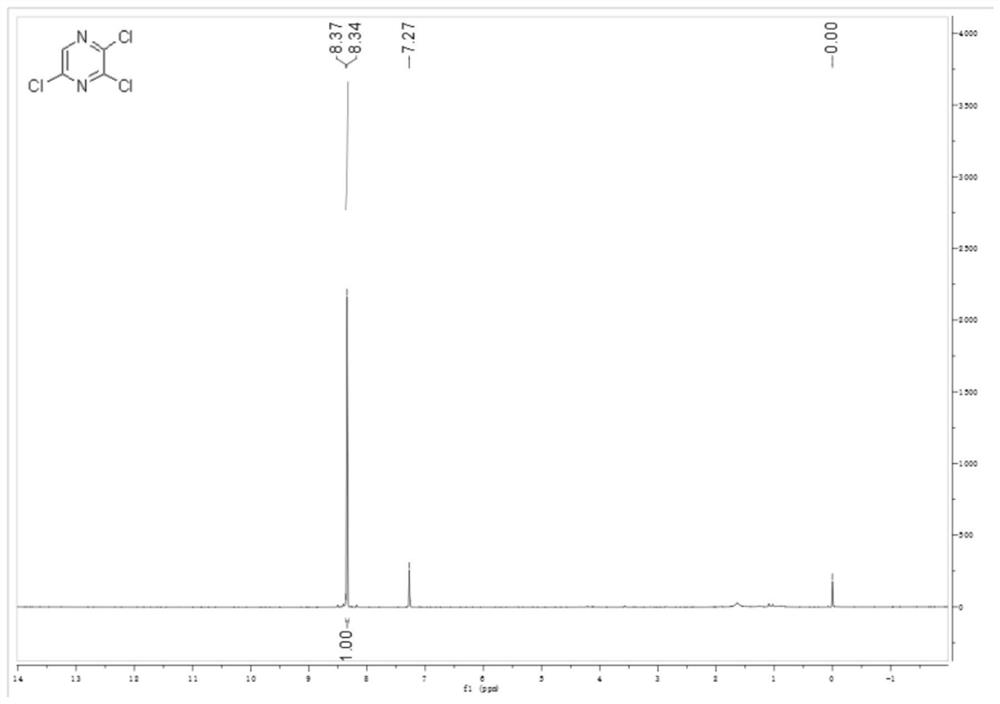
[0044](1) Weigh 100g (0.873mol) of dry 2-chloropyrazine into a 1-liter three-neck flask, replace the nitrogen, add 57.4g (0.786mol) of freshly steamed DMF, control the temperature in an ice bath, and add sulfuryl chloride dropwise at a temperature below 20°C 589g (4.36mol), gas is released, and the addition is completed in about 2 hours; keep at about 20°C for 30 minutes, then raise the temperature to 70°C for 4 hours; HPLC monitors the end of the reaction. Stop the reaction, cool to room temperature, slowly pour the system into 1500g of crushed ice with stirring, extract 300mL of methyl tert-butyl ether each time, extract 3 times in total, dry with anhydrous sodium sulfate, and spin dry to obtain light yellow flaky crystals 125.5 g, purity 97.2%, yield 76.43%. 1 HNMR spectrum see figure 1 .
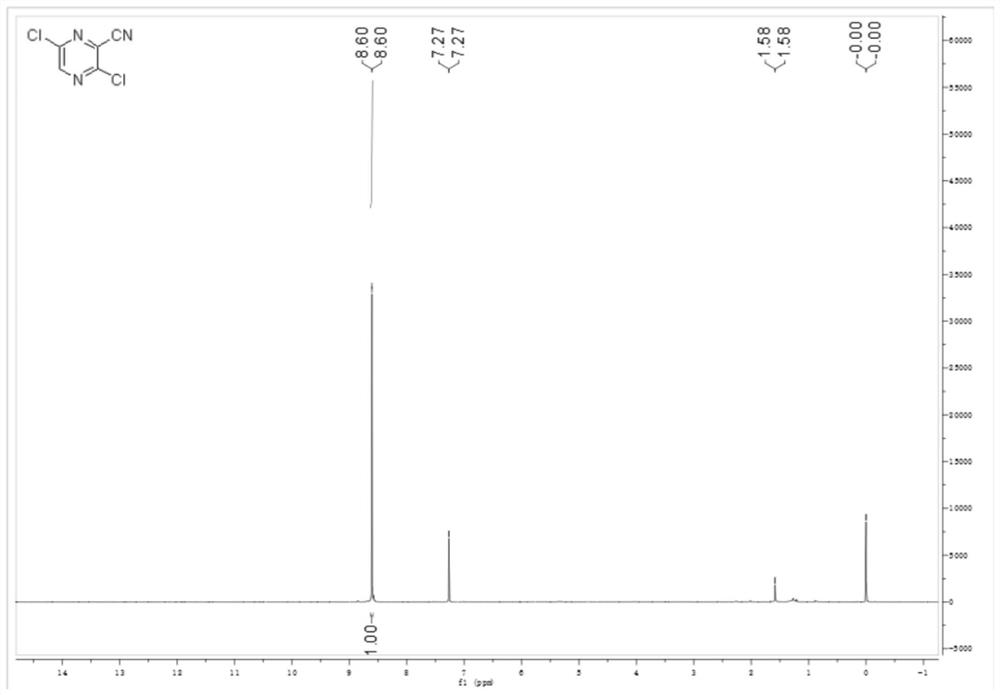
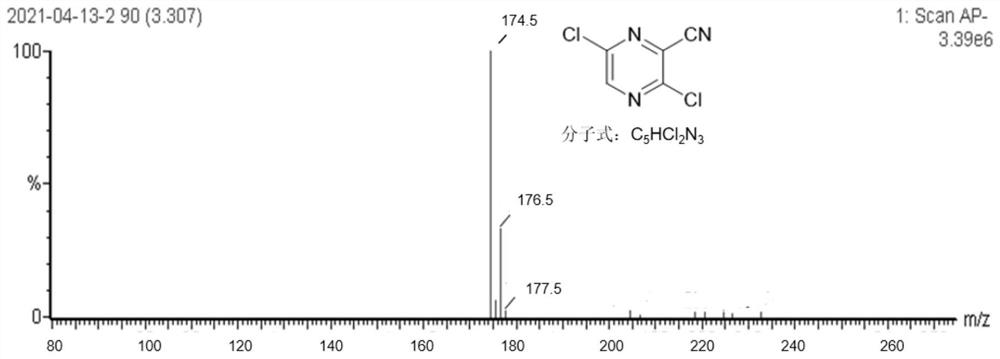
[0045] (2) Weigh 50g (0.275mol) 2,3,5-trichloropyrazine into a 500ml three-necked flask, add 0.3g (0.55mmol) 1,3-bis(diphenylphosphinopropane) nickel dichloride and 3.3g (8.25mmol) PE...
Embodiment 2
[0047] The other steps are the same as in Example 1, except that 1,3-bis(diphenylphosphinopropane)nickel dichloride is replaced by an equimolar amount of [1,1'-bis(diphenylphosphino)ferrocene]dichloride nickel chloride. The purity of the target product 3,6-dichloro-2-cyanopyrazine was 97.4%, and the total yield was 55.38%.
Embodiment 3
[0049] Other steps are the same as in Example 1, except that PEG400 is replaced by PEG800 in an equimolar amount. The purity of the target product 3,6-dichloro-2-cyanopyrazine was 98.1%, and the total yield was 56.72%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com