Method for preparing benzodinitrogen compound by using gold complex
A technology of gold complexes and benzodiazepines, which is applied in the field of preparation of benzodiazepines, can solve problems such as catalyst instability, influence on catalytic efficiency, and long reaction time, and achieve simple and green preparation methods, wide application prospects, The effect of low catalyst dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
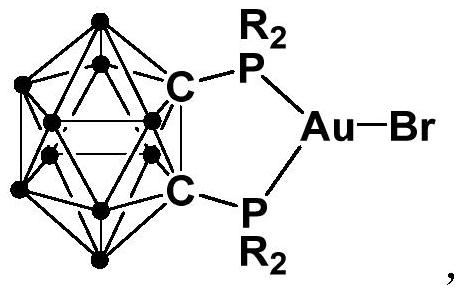
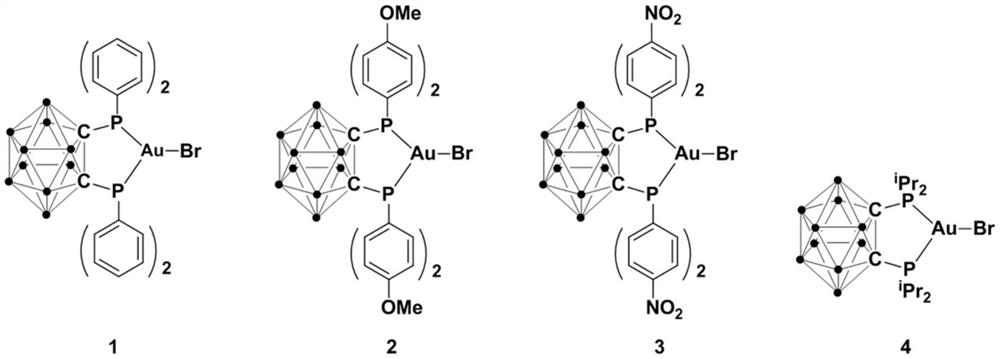
[0031] Synthesis of gold complex 1:
[0032] At 0°C, add n-BuLi (2.2mmol) n-hexane solution dropwise to the ortho carborane o-C 2 B 10 h 12 (1.0mmol) in ether solution, continue stirring for 30 minutes after the dropwise addition, slowly rise to room temperature and continue to react for 30 minutes, then add halophosphine ClPPh 2 (2.2mmol), continue to react at room temperature for 2 hours, then add AuBr (1.0mmol) to the reaction system and continue to react at room temperature for 3 hours, after the reaction is over, stand and filter, dry the solvent under reduced pressure, and the crude product obtained is washed with diethyl ether , drained to obtain target product 1 (yield 81%), its reaction formula is:
[0033]
[0034] 1 H NMR (400MHz, CDCl 3 , 25°C): δ=7.69-7.63 (m, 8H), 7.51-7.42 (m, 12H). Elemental Analysis Theoretical Value C 26 B 10 h 30 P 2 AuBr: C 39.56, H 3.83; Found: C 39.51, H 3.90.
Embodiment 2
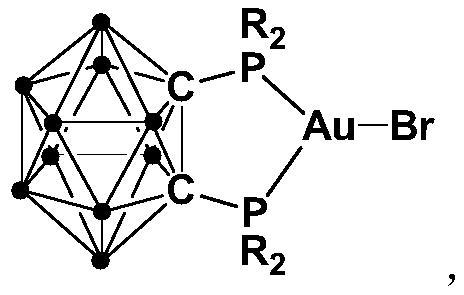
[0036] Synthesis of gold complex 2:
[0037] At 0°C, n-BuLi (2.5 mmol) in n-hexane was added dropwise to the ortho carborane o-C 2 B 10 h 12 (1.0mmol) ether solution, continue to stir for 30 minutes after the dropwise addition, slowly rise to room temperature and continue to react for 30 minutes, then add halophosphine ClP (4-MeO-C 6 h 4 ) 2 (2.5mmol), continue to react at room temperature for 2 hours, then add AuBr (1.0mmol) to the reaction system and continue to react at room temperature for 5 hours, after the reaction is over, stand and filter, and dry the solvent under reduced pressure, and the crude product obtained is washed with diethyl ether , drained to obtain the target product 2 (yield 83%), the reaction formula is:
[0038]
[0039] 1 H NMR (400MHz, CDCl 3 , 25°C): δ=7.80-7.73 (m, 8H), 7.63-7.55 (m, 8H), 3.37 (s, 12H). Elemental Analysis Theoretical Value C 30 B 10 h 38 o 4 P 2 AuBr: C 39.62, H 4.21; Found: C 39.66, H 4.28.
Embodiment 3
[0041] Synthesis of gold complex 3:
[0042] At 0°C, add n-BuLi (2.3mmol) n-hexane solution dropwise to the ortho carborane o-C 2 B 10 h 12 (1.0mmol) in ether solution, continue stirring for 30 minutes after the dropwise addition, slowly rise to room temperature and continue to react for 30 minutes, then add halophosphine ClP(4-NO 2 -C 6 h 4 ) 2 (3.0mmol), continue to react at room temperature for 2 hours, then AuBr (1.0mmol) is added to the reaction system at room temperature and continue to react for 4 hours, after the reaction is completed, stand and filter, dry the solvent under reduced pressure, and the crude product obtained is washed with ether , drained to obtain the target product 3 (yield 77%), the reaction formula is:
[0043]
[0044] 1 H NMR (400MHz, CDCl 3 , 25°C): δ=7.86-7.77 (m, 8H), 7.69-7.61 (m, 8H). Elemental Analysis Theoretical Value C 26 B 10 h 26 N 4 o 8 P 2 AuBr: C 32.21, H 2.70, N 5.78; Found: C 32.30, H 2.66, N 5.82.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com