Method for transfecting immune cells by lentivirus
A technology of lentiviral transfection and immune cells, applied in blood/immune system cells, viruses, animal cells, etc., can solve the problems of inaccessibility and high transfection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Materials and Methods
[0066] 1. Obtaining of PBMC (peripheral blood mononuclear cells) and CBMC (cord blood mononuclear cells):
[0067] Take peripheral blood or umbilical cord blood from a healthy person, add an equal volume of normal saline to dilute at room temperature, and slowly add it to the lymphocyte separation solution along the wall of the centrifuge tube, so that the ratio of diluted blood to lymphocyte separation solution is 2:1. 2000rpm (equivalent to 872g), centrifuged for 20min, and divided into four layers from the bottom of the tube to the liquid surface after centrifugation, followed by erythrocyte and granulocyte layer, liquid separation layer, mononuclear cell layer, and plasma layer. Aspirate the mononuclear cell layer into a new centrifuge tube, add physiological saline not less than 3 times the volume of the mononuclear cell layer to wash the cells, 2000rpm, 5min, twice, and count the cells. Obtain PBMC or CBMC.
[0068] 2. Sorting and culturi...
Embodiment 2
[0128] Example 2 lentivirus transfection of αβT cells derived from peripheral blood
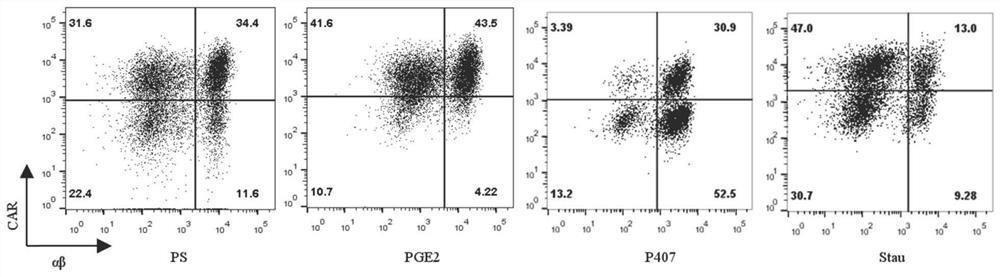
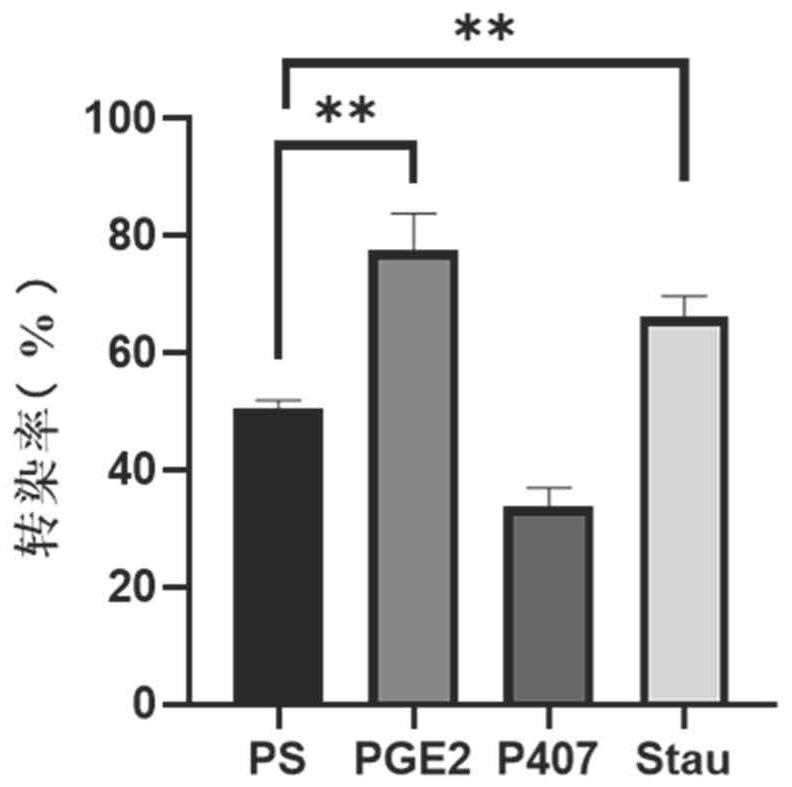
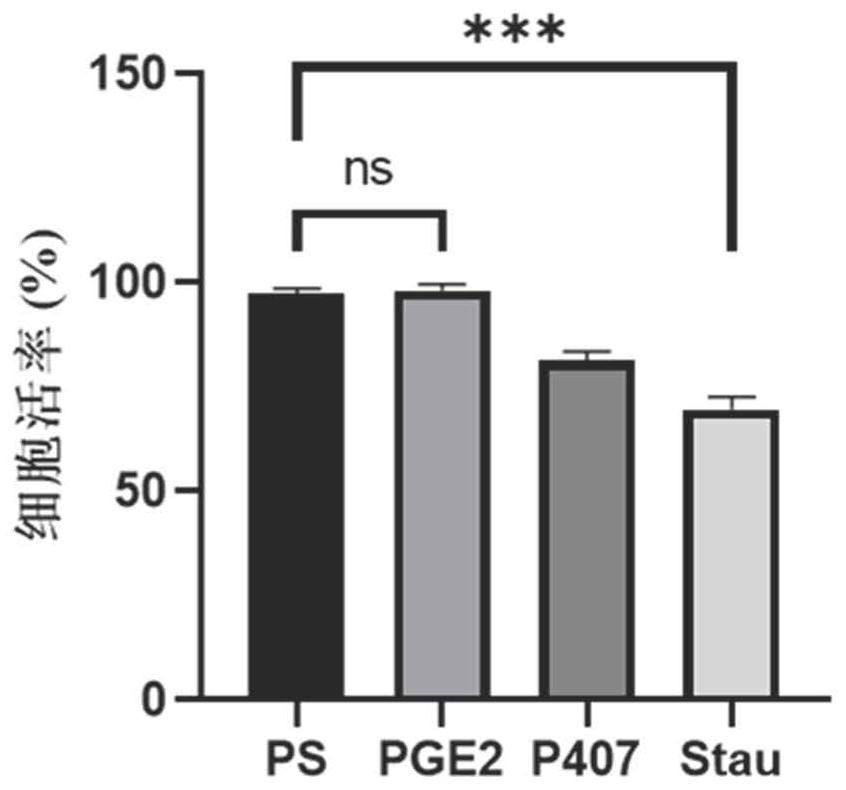
[0129] The peripheral blood-derived αβT cells cultured for 2 days in Example 1 were used and divided into PS group, PGE2 group, P407 group and Stau group. According to 3×10 5 Inoculate each well in a 24-well plate, add serum-free medium (KBM581+200IU / ml IL-2) to each well, add the virus concentrate U6-SR8-MND1904 prepared in Example 1 according to the amount of MOI=2, PS Group, PGE2 group, P407 group and Stau group were added different transfection aids according to the concentration of PS 8μg / ml, PGE21μM, P407100μg / mL, Stau 200nM, and the final volume of the system was 500ul / well. Centrifuge at 2000rpm at 35°C for 2h, take it out and put it in 37°C, 5% CO 2 For culture, the medium is KBM581+200IU / ml IL-2, and tested after 96h.
[0130] The transfection efficiency was detected by flow cytometry, and the specific antibodies used were EGFR-APC, CD3-APC-cy7, CD4-FITC, CD8-PB, 7AAD, and SA-PE....
Embodiment 3
[0133] Example 3 lentivirus transfection of peripheral blood-derived γδT cells
[0134] The peripheral blood-derived γδT cells cultured for 6 days in Example 1 were used and divided into PS group, PGE2 group, P407 group and Stau group. According to 1×10 6 Cells were inoculated at a concentration of 1 / mL, the medium was serum-free medium (KBM581+1000IU / mlIL-2), and the virus concentrate U6-SR8-MND1904 prepared in Example 1 was added according to the dosage of MOI=50, PS group, PGE2 group, P407 group and Stau group were added with different transfection-assisted reagents according to the concentrations of PS 8 μg / ml, PGE2 1 μM, P407 100 μg / mL, and Stau 200 nM, and the final volume of the system was 500 μl / well. The incubation was continued after centrifugation at 35°C for 2 hours. The next day, the medium was replaced with the expansion medium described in Example 1. 96h after transfection, samples were taken for detection.
[0135] The transfection efficiency was detected b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com