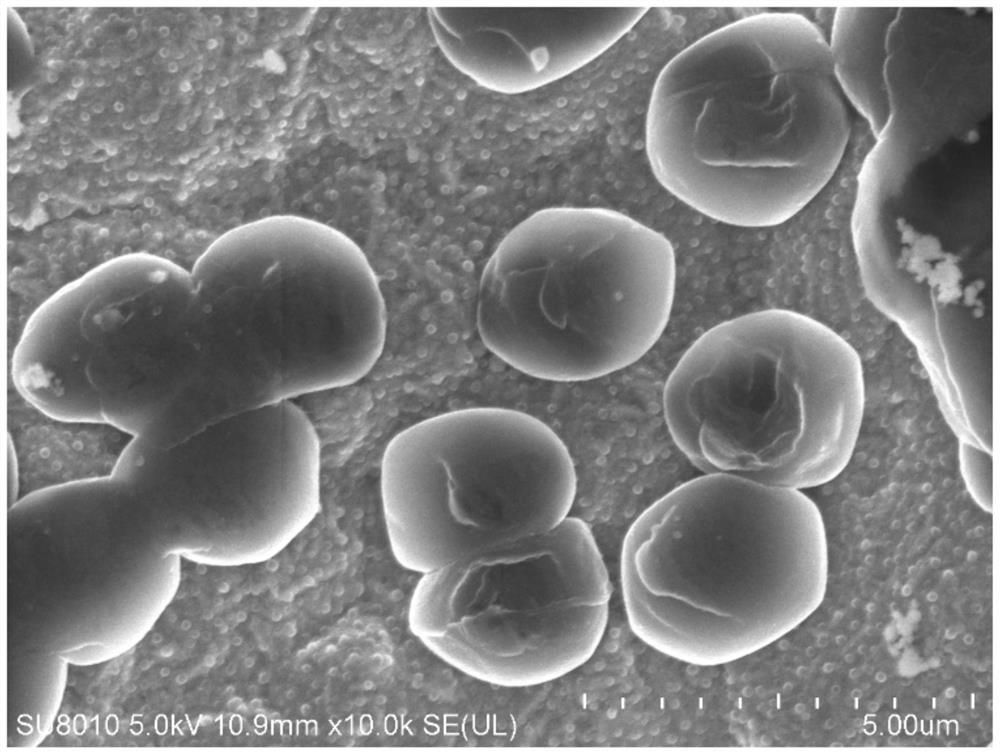
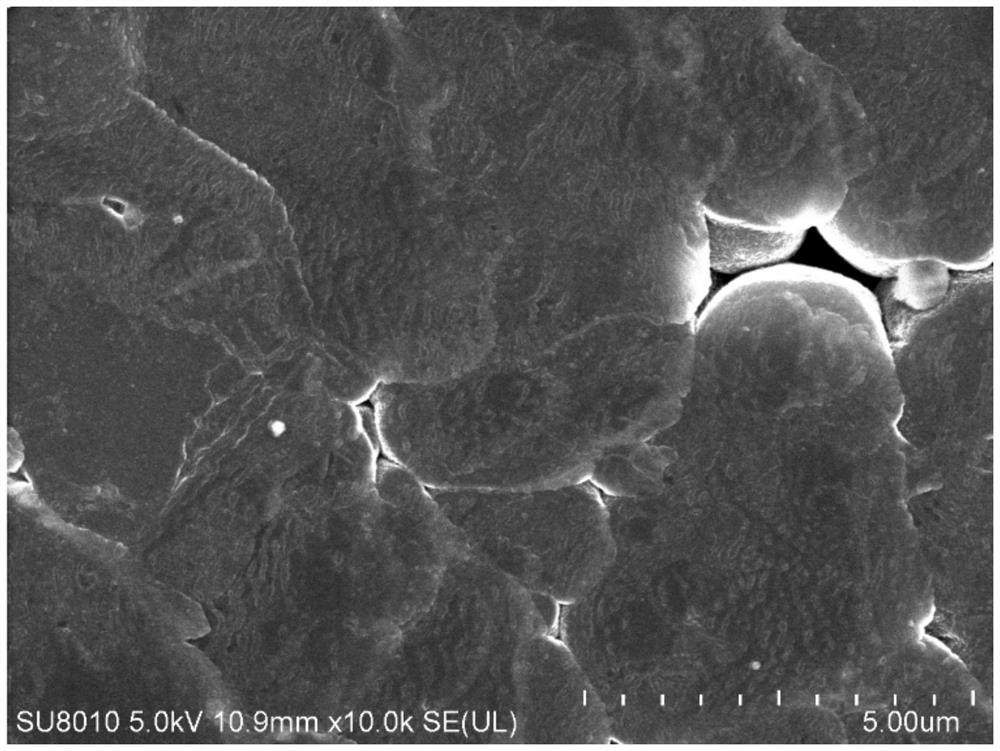
Carbonic ester electrolyte and metal lithium battery
A technology of carbonates and electrolytes, applied in electrolytes, secondary batteries, secondary battery repair/maintenance, etc., can solve the problems of low oxidation cut-off voltage, low nitrate solubility, and inability to match high-voltage positive electrode materials, etc., to achieve improved The effect of solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Electrolyte preparation:
[0039] Prepare 10 mL of electrolyte in an argon protective glove box at room temperature.
[0040] First, 0.01mol LiPF 6 Add to the mixed solvent of fluoroethylene carbonate, ethylene carbonate and diethyl carbonate to obtain a mixed solution, wherein the volume ratio of fluoroethylene carbonate: ethylene carbonate: diethyl carbonate is 2:9:9 . Add 0.001 mol of rubidium nitrate and 0.001 mol of 18-crown-6 into the mixed solution, stir thoroughly for 1 hour to obtain a uniform electrolyte, wherein the cation (Rb) in rubidium nitrate + The ratio of the diameter D1 of ) to the diameter D2 of the central cavity of 18-crown-6 is 1.
[0041] Preparation of metal lithium battery:
[0042]Copper foil was used as the positive electrode, lithium foil was used as the negative electrode, and Celgard separator was used as the separator, and 60 μL of the electrolyte solution prepared above was used to assemble a button cell.
Embodiment 2
[0044] The difference between Example 2 and Example 1 is that in the preparation of the electrolyte, rubidium nitrate is replaced by potassium nitrate.
Embodiment 3
[0046] The difference between Example 3 and Example 1 is that in the preparation of the electrolyte, rubidium nitrate is replaced by sodium nitrate, and 18-crown-6 is replaced by 15-crown-5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com