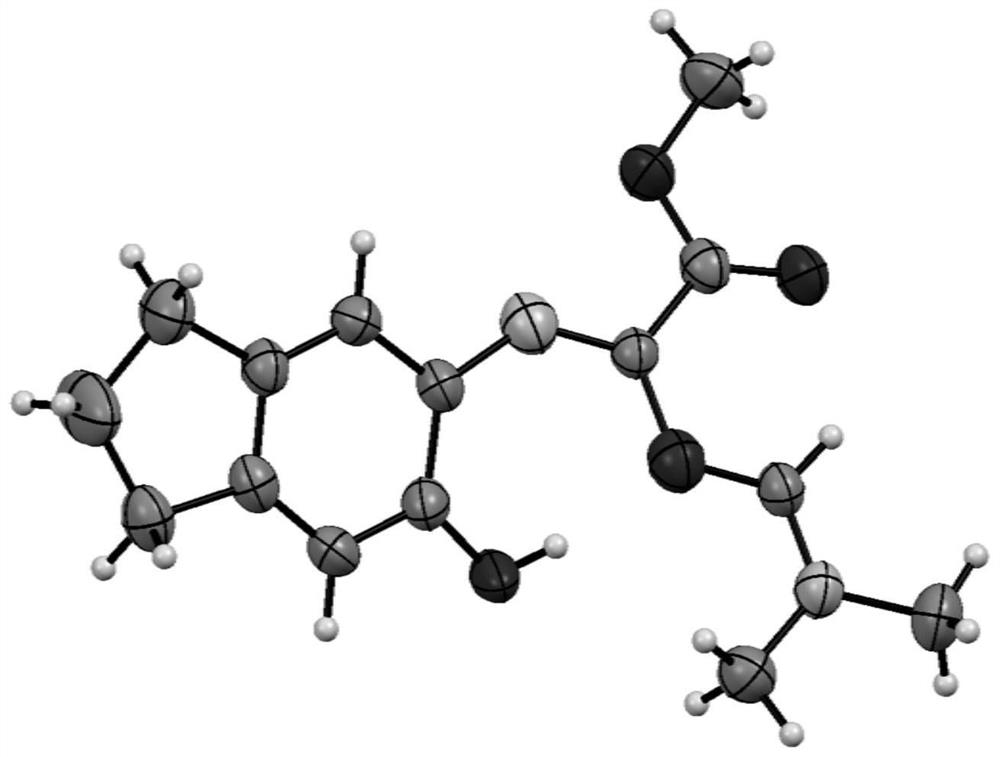
Synthesis method of conjugated alkenyl amidine compound
A synthesis method and compound technology, which is applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of poor universality of substrates, and achieve the effects of easy separation and purification, stable products, and reduced environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~24
[0061] The following examples 1 to 24 were all reacted under optimal reaction conditions. The specific reaction equation is as follows, mainly to investigate the yield of different substrates under optimal conditions:
[0062] Concrete reaction formula is as follows:
[0063]
[0064] The specific operation steps are:
[0065] Potassium fluoride (0.046g, 4.0equiv) and 18-crown-6 (0.052g, 1.0equiv) were sequentially added into a clean and dry Schlenk tube, the double exhaust tube was evacuated and replaced with nitrogen for three times, and the The isonitrile compound (1.0 equiv), Kobayashi aryne precursor derivative (1.5 equiv) and DMF (1.0 mL) were added at the same time. The mixture was stirred at room temperature for 5 h, and a sample was taken for detection. TLC monitored that the reaction raw materials disappeared completely. Add 1M H to the reaction system 2 O was diluted and extracted three times with ethyl acetate, the organic phases were combined, washed with sa...
Embodiment 1
[0067] Compound 1: The yield is 85%.
[0068]
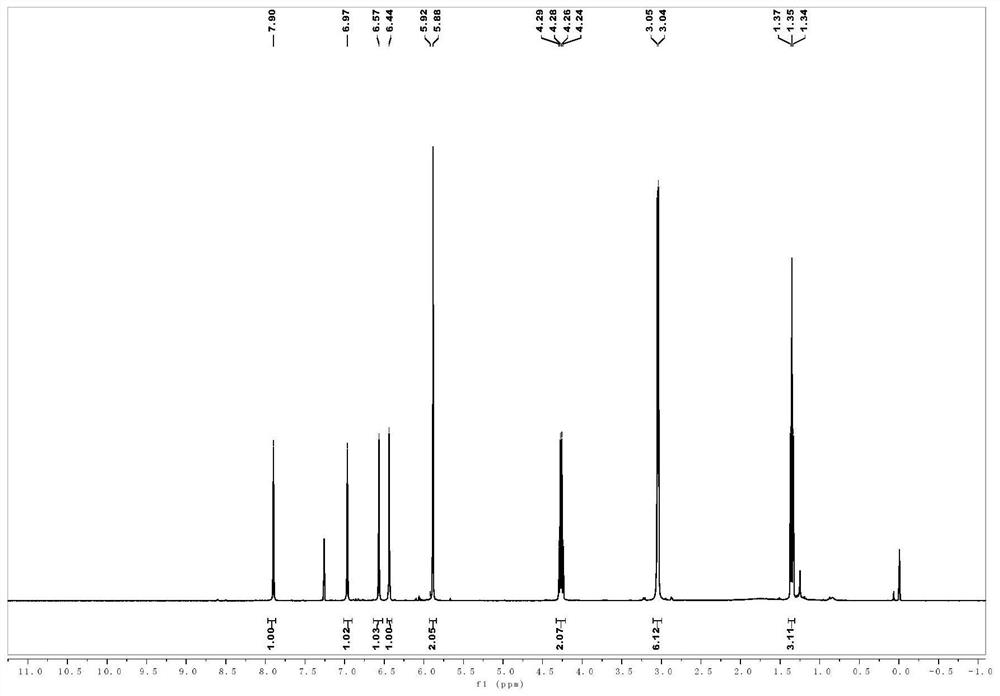
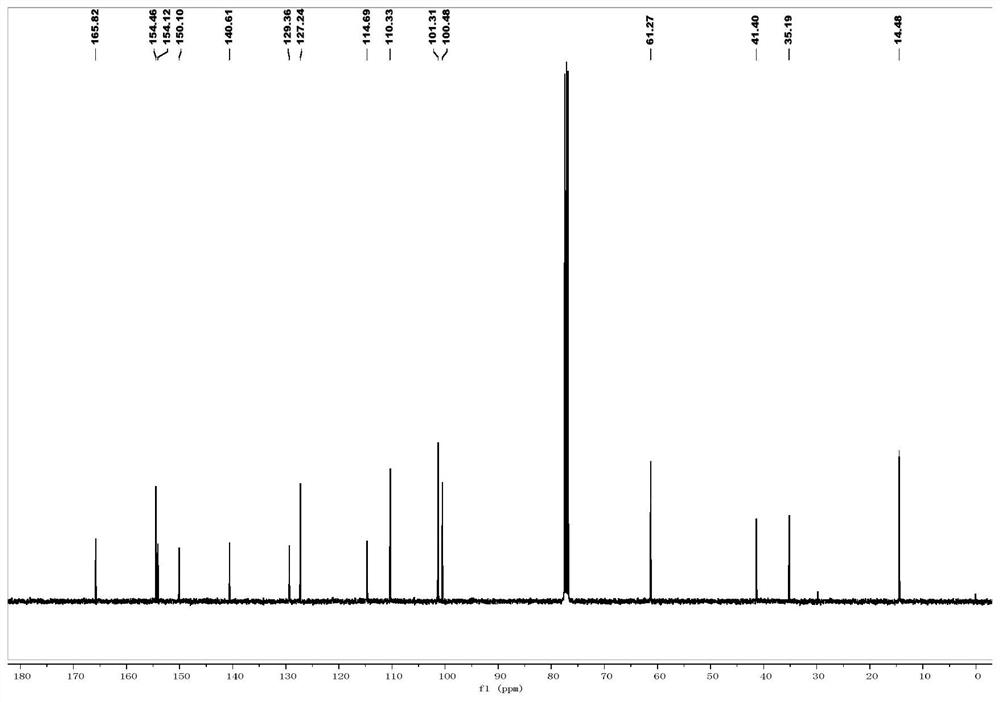
[0069] 1 H NMR (400MHz, CDCl 3 -d) δ7.91(s,1H),7.21(ddd,J=8.5,7.2,1.7Hz,1H),7.14(dd,J=7.8,1.7Hz,1H),7.08(s,1H),6.91 (dd, J=8.2,1.3Hz,1H),6.80(td,J=7.4,1.3Hz,1H),4.29(q,J=7.1Hz,2H),3.08(s,3H),3.05(s, 3H), 1.37(t, J=7.1Hz, 3H); 13 CNMR (100MHz, CDCl 3 -d) δ165.4, 158.5, 154.9, 132.4, 130.6, 125.9, 125.5, 123.8, 123.4, 119.0, 61.7, 41.5, 35.2, 14.4; HRMS(ESI) m / z calcd for C 14 h 18 N 2 o 3 + [M+H] + 263.1390,found263.1390.
Embodiment 2
[0071] Compound 2: The yield is 72%.
[0072]
[0073] 1 H NMR (400MHz, CDCl 3-d) δ7.88(s,1H),7.09(s,1H),6.99(s,1H),6.79(s,1H),4.28(q,J=7.1Hz,2H),3.04(d,J =13.9Hz, 6H), 2.83(dt, J=21.6, 7.4Hz, 4H), 2.04(p, J=7.4Hz, 2H), 1.36(t, J=7.2Hz, 3H); 13 C NMR (100MHz, CDCl3-d) δ166.0, 156.2, 154.6, 148.0, 134.8, 130.3, 127.9, 127.8, 120.7, 114.8, 61.26, 41.3, 35.1, 33.2, 31.8, 26.0, 14.5; HRMS (ESI) m / z calcd for C 17 h 22 N 2 o 3 + [M+H] + 303.1703,found 303.1706.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com