Method for synthesizing acetylenic acid by using terminal alkyne and carbon dioxide
A technology of carbon dioxide and alkynoic acid, which is applied in the direction of organic chemical methods, chemical instruments and methods, and the preparation of organic compounds, can solve the problems of complex catalyst synthesis, harsh reaction conditions, and high catalytic costs, and achieve industrial production application prospects and good, The effect of mild reaction conditions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] A kind of method utilizing terminal alkyne and carbon dioxide to synthesize alkynoic acid, comprises the steps:
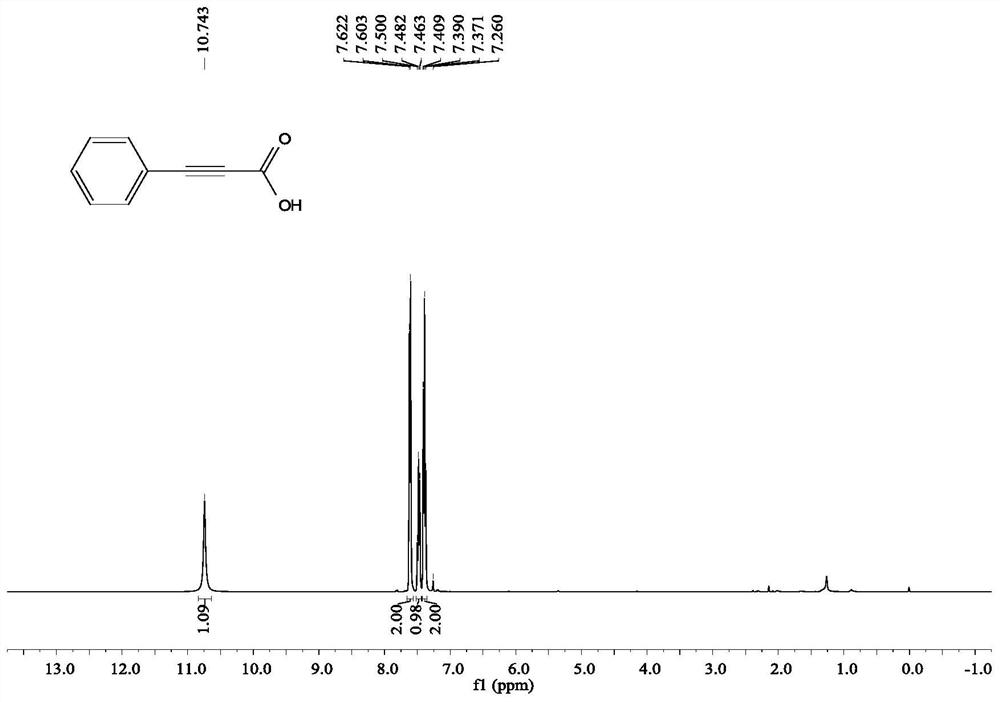
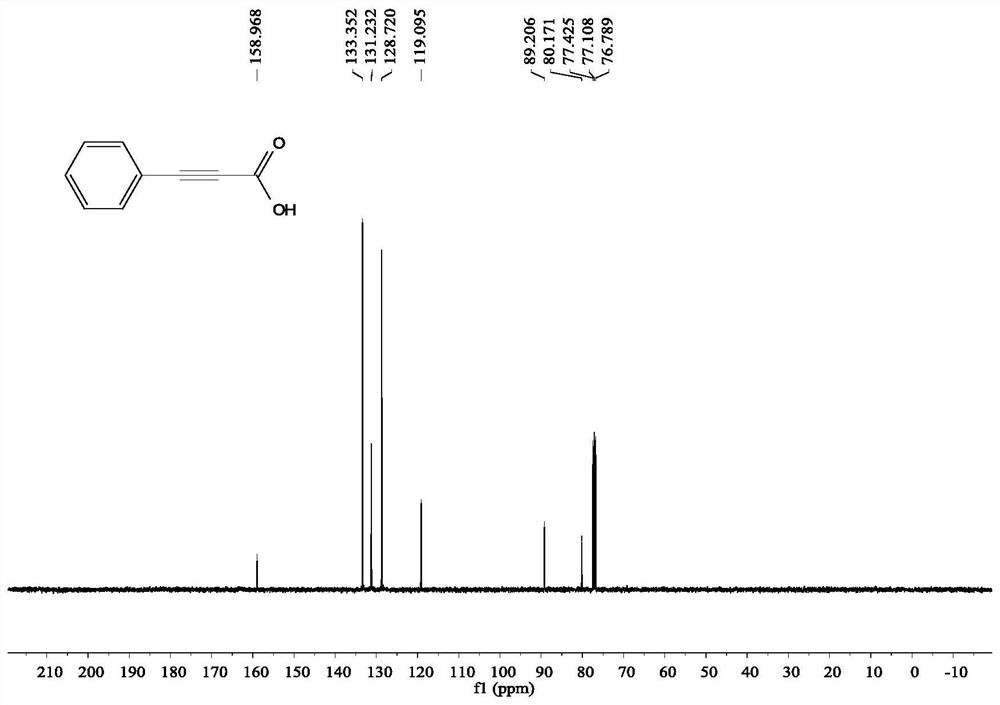
[0054] Add 4 ml of dimethyl sulfoxide, 1 mmol of phenylacetylene, and 2 mmol of cesium carbonate into the reaction tube, pump and ventilate the reaction tube 3 times, and fill with CO 2 , filled with the CO 2 The gas pressure of the rear reaction tube is 1 atmosphere, and the reaction is stirred and reacted for 24 hours under the condition of carbon dioxide atmosphere and 60 ° C, the stirring rate is 800 rpm, the stirring is stopped, cooled to room temperature, and 5 ml of sodium hydroxide of 2 moles per liter is added to the reaction solution solution, add 5 ml of water, extract 4 times with ethyl acetate, separate the layers, take the water layer, acidify the water layer with 2 moles per liter of hydrochloric acid to pH = 1, then extract with ethyl acetate, take the organic layer, the organic layer Wash with saturated brine and dry over magnesium sulfate,...
Embodiment 2
[0056] A kind of method utilizing terminal alkyne and carbon dioxide to synthesize alkynoic acid, comprises the steps:
[0057] Add 4 ml of dimethyl sulfoxide, 1 mmol of phenylacetylene, and 1.5 mmol of cesium carbonate into the reaction tube, pump and ventilate the reaction tube 3 times, and fill with CO 2 , filled with the CO 2 The gas pressure of the rear reaction tube is 1 atmosphere, and the reaction is stirred and reacted for 24 hours under the condition of carbon dioxide atmosphere and 60 ° C, the stirring rate is 800 rpm, the stirring is stopped, cooled to room temperature, and 5 ml of sodium hydroxide of 2 moles per liter is added to the reaction solution solution, add 5 ml of water, extract 4 times with ethyl acetate, separate the layers, take the water layer, acidify the water layer with 2 moles per liter of hydrochloric acid to pH = 1, then extract with ethyl acetate, separate the layers, and take the organic layer , the organic layer was washed with saturated bri...
Embodiment 3
[0059] A kind of method utilizing terminal alkyne and carbon dioxide to synthesize alkynoic acid, comprises the steps:
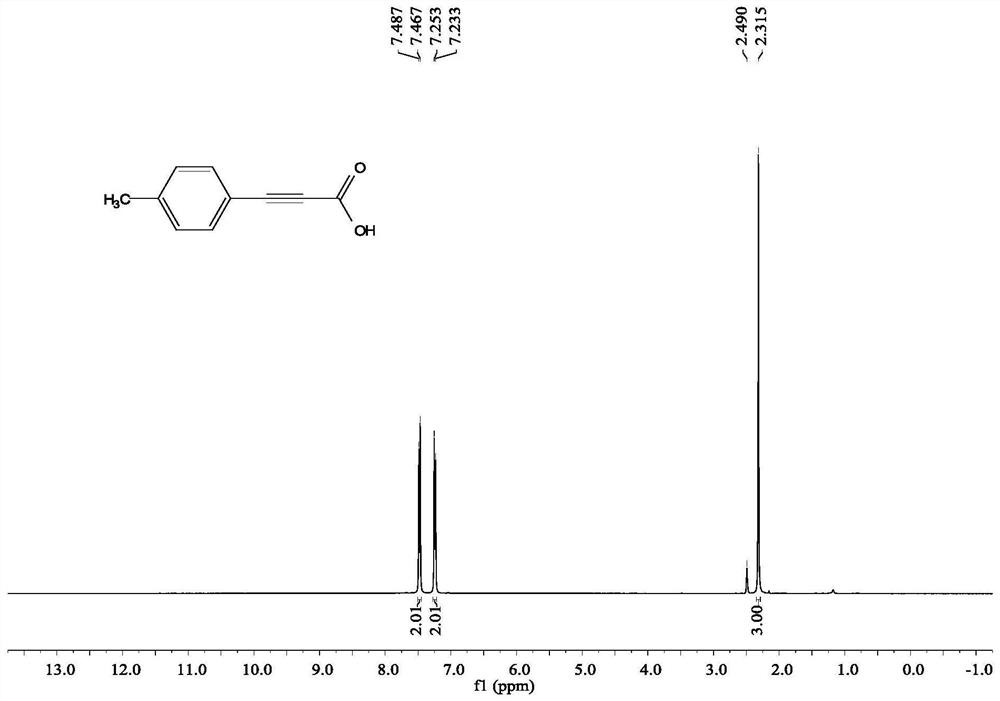
[0060] Add 4 ml of dimethyl sulfoxide, 1 mmol of phenylacetylene, and 2.5 mmol of cesium carbonate into the reaction tube, pump and ventilate the reaction tube 3 times, and fill with CO 2 , filled with the CO 2 The gas pressure of the rear reaction tube was 1 atmosphere, and the reaction was stirred and reacted for 24 hours under the conditions of carbon dioxide atmosphere and 60° C., the stirring rate was 800 rpm, the stirring was stopped, cooled to room temperature, the reaction liquid was added with water, extracted 4 times with ethyl acetate, and divided solution, take the water layer, and acidify the water layer to pH = 1 with 2 moles per liter of hydrochloric acid, then extract with ethyl acetate, take the organic layer, wash the organic layer with saturated brine and dry on magnesium sulfate, filter the filtrate, and reduce pressure Concentration gav...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com