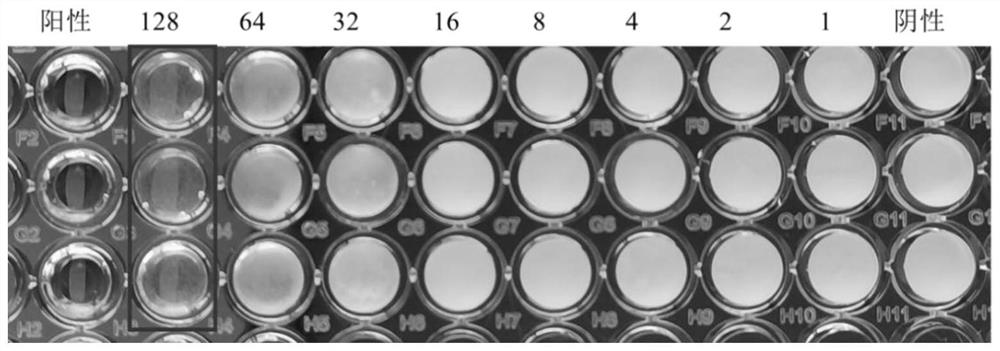
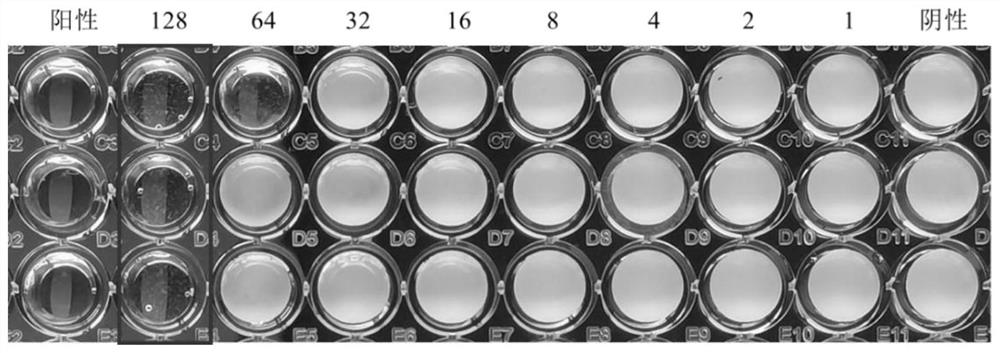
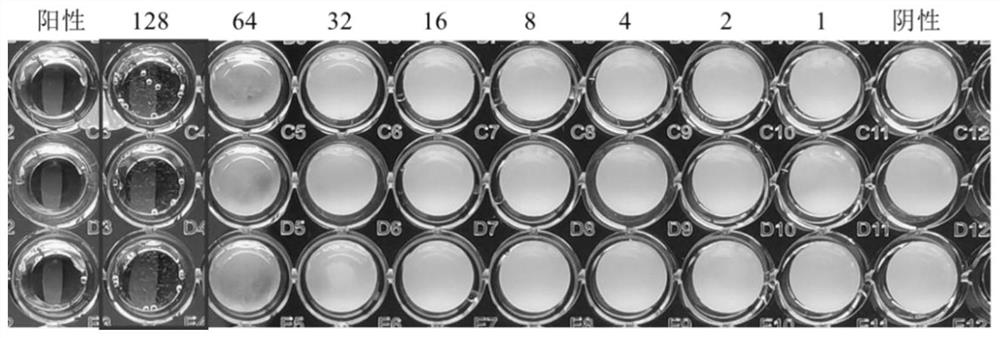
2-amino selenophenes compound and synthesis method and application thereof
A synthesis method and compound technology, applied in the direction of organic chemistry, drug combination, antibacterial drugs, etc., can solve the problems of unstable reaction, harsh reaction conditions, cumbersome reaction operation, etc., and achieve cheap and easy solubility and substrate adaptability The effect of strong sex and simple raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Preparation of compound 1
[0042]
[0043] Take 98mg of cyclohexanone and 114mg of ethyl cyanoacetate (molar ratio 1:1) in a 50mL one-necked flask, add 20mL of ethanol to dissolve, oscillate at 45°C and 40KHz for 20min, then add Na 2 Se 225mg (molar ratio 1:1:1.8), react under nitrogen protection, 45°C, 40KHz ultrasonic vibration. At the end of the reaction, the reaction solution became turbid. The reaction solution was poured into cold water, allowed to stand, and filtered to obtain a crude product, which was further purified by recrystallization with methanol to obtain 104.8 mg of compound 1 with a yield of 38.5%.
[0044] 1 H-NMR (400MHz, DMSO-d 6 )δ(ppm):8.52(2H,s),4.25(2H,m),2.29(2H,t),2.02(2H,t),1.73(2H,m),1.69(2H,m),1.35( 3H,t). 13 C-NMR (400MHz, DMSO-d 6 )δ (ppm): 166.0, 154.6, 145.0, 142.1, 109.0, 63.0, 32.1, 30.2, 28.1, 23.6, 15.8
Embodiment 2
[0046] Preparation of Compound 2
[0047]
[0048] Take 84mg of cyclopentanone and 114mg of ethyl cyanoacetate (molar ratio 1:1) in a 50mL single-necked flask, add 20mL of ethanol to dissolve, ultrasonically shake at 30°C and 40KHz for 20min, then add Na 2 Se 225mg (molar ratio 1:1:1.8), react under nitrogen protection, 30°C, 40KHz ultrasonic vibration. At the end of the reaction, the reaction solution became turbid, and the reaction solution was poured into cold water, allowed to stand, and filtered to obtain a crude product, which was further purified by recrystallization with methanol to obtain 79.8 mg of compound 2 with a yield of 30.9%.
[0049] 1 H-NMR (400MHz, DMSO-d 6 )δ (ppm): 8.49 (2H, s), 4.17 (2H, m), 2.35 (2H, t), 2.24 (2H, t), 1.92 (2H, m), 1.35 (3H, t); 13 C-NMR (400MHz, DMSO-d 6 )δ (ppm): 165.8, 149.4, 145.6, 141.3, 110.2, 60.2, 44.7, 42.5, 25.2, 12.9
Embodiment 3
[0051] Preparation of compound 3
[0052]
[0053] Take 112mg of cycloheptanone and 114mg of ethyl cyanoacetate (molar ratio 1:1) in a 50mL single-necked flask, add 20mL of ethanol to dissolve, oscillate ultrasonically at 60°C and 40KHz for 20min, then add Na 2 Se 187.5mg (molar ratio 1:1:1.5), react under nitrogen protection, 60°C, 40KHz ultrasonic vibration. At the end of the reaction, the reaction solution became turbid. The reaction solution was poured into cold water, allowed to stand, and filtered to obtain a crude product, which was further purified by recrystallization with methanol to obtain 84.2 mg of compound 3 with a yield of 29.4%.
[0054] 1 H-NMR (400MHz, DMSO-d 6 )δ(ppm):8.53(2H,s),4.29(2H,m),2.05(2H,t),1.90(2H,t),1.62(2H,m),1.84(2H,m),1.49( 2H,m),1.40(3H,t); 13 C-NMR (400MHz, DMSO-d 6 )δ (ppm): 163.1, 151.2, 142.5, 141.8, 110.0, 60.5, 31.8, 31.5, 25.6, 22.3, 15.2
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com