Lycoris aurea 1-aminocyclopropane-1-carboxylic acid synthetase as well as coding gene and application thereof
A technology of aminocyclopropane and synthase, applied in the fields of biotechnology and plant biology, can solve the problems of difficulty in practical production and high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Cloning of embodiment 1, 1-aminocyclopropane-1-carboxylic acid synthetase LaACS coding gene
[0038] The two primers synthesized respectively have the nucleotide sequences of SEQ ID NO:3 and SEQ ID NO:4 in the sequence listing.
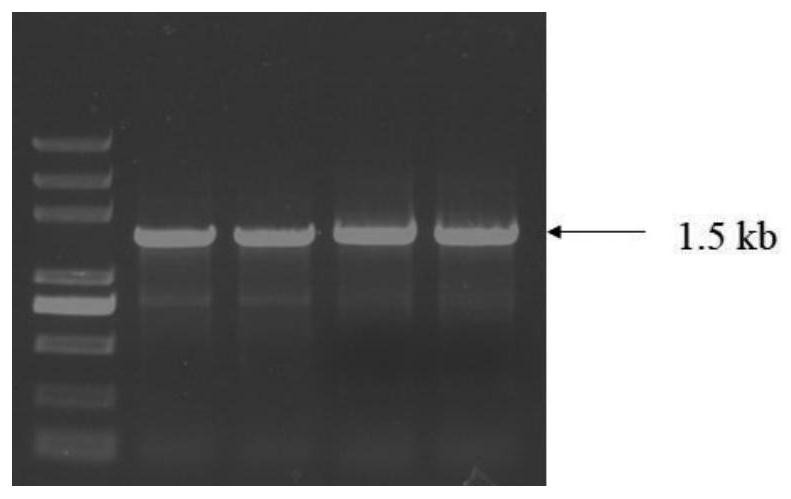
[0039] Using the cDNA obtained by reverse transcription of the RNA extracted from Hudixiao as a template, PCR was performed using the above two primers SEQ ID NO:3 and SEQ ID NO:4. The DNA polymerase is Super-Fidelity DNA polymerase from Nanjing Nuoweizan Biotechnology Co., Ltd. The PCR amplification program was: 95°C for 5min; 94°C for 10s, 56°C for 10s, 72°C for 1min, 32 cycles; 72°C for 10min, 10°C for heat preservation. The PCR products were detected by agarose gel electrophoresis, and the amplification results were as follows: figure 1 . Excise a DNA band of the same size as the target. DNA was recovered from the agarose gel using a DNA purification kit (Beijing Biotek Biotechnology Co., Ltd.). The recovered PCR product was cloned int...
Embodiment 2
[0041] Embodiment 2, the construction of Escherichia coli and Corynebacterium recombinant expression vector of LaACS gene
[0042] (1) Construction of expression vector pECXK-LaACS
[0043] Two primers respectively having the nucleotide sequences of SEQ ID NO:5 and SEQ ID NO:6 in the sequence listing were synthesized. Two restriction sites, BamHI and SalI, and homologous recombination sequences were set at the 5' ends of the synthetic primers SEQ ID NO:5 and SEQ ID NO:6, respectively, and 6 histidines were introduced at the N-terminus, and pMD19- T-LaACS was used as template for PCR amplification. The PCR amplification procedure is the same as in Example 1. The PCR amplified product was detected by agarose gel electrophoresis, separated, and recovered by cutting the gel. Then, it was ligated into the pECXK-99E vector digested with BamHI and SalI by homologous recombination, and a His expression tag was introduced into the N-terminal for subsequent use. protein purification....
Embodiment 3
[0046] Induced expression of embodiment 3, LaACS protein
[0047] The prokaryotic expression of protein will be affected by induction time, induction temperature, promoter strength and host. Therefore, the prokaryotic expression of the protein can be optimized by changing the induction conditions and using different expression vectors and host bacteria. The successfully constructed recombinant expression vector pECXK-His-LaACS was transformed into Escherichia coli BL21(DE3) and Corynebacterium ammoniagenes 21170 respectively, and the recombinant expression vector pET29a-LaACS was transformed into Escherichia coli BL21(DE3). The obtained recombinant strains BL21(DE3) / pECXK-His-LaACS, BL21(DE3) / pET29a-LaACS and Corynebacterium ammoniagenes ATCC21170 / pECXK-His-LaACS were stored in a -80°C refrigerator for later use.
[0048] (1) Escherichia coli medium formula: the selected induction medium formula (1L) is 23g Na 2 HPO 4 , 5g KH 2 PO 4 , 2.5g NaCl, 5.0g (NH 4 ) 2 SO 4 , 0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com