Compositions and methods to promote host defense and stimulate, expand, and/or reset t cell repertoires
A technology of compositions and derivatives, which can be used in drug combinations, active ingredients of hydroxy compounds, animal feeds, etc., can solve the problems of respiratory or oral allergies without protective effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
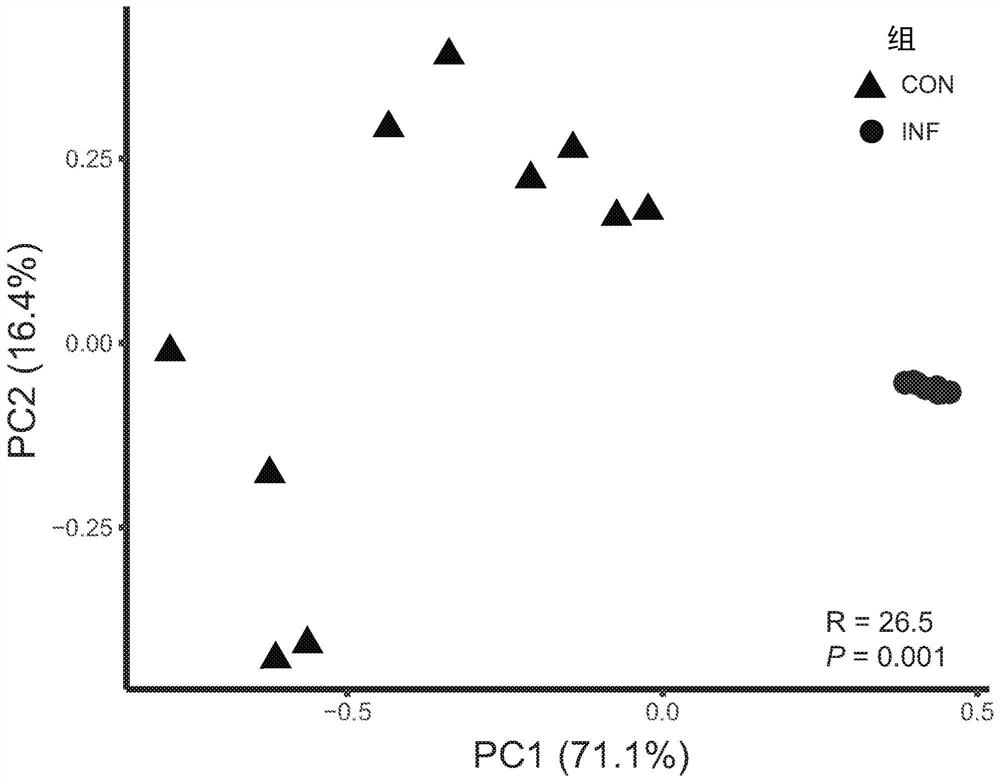
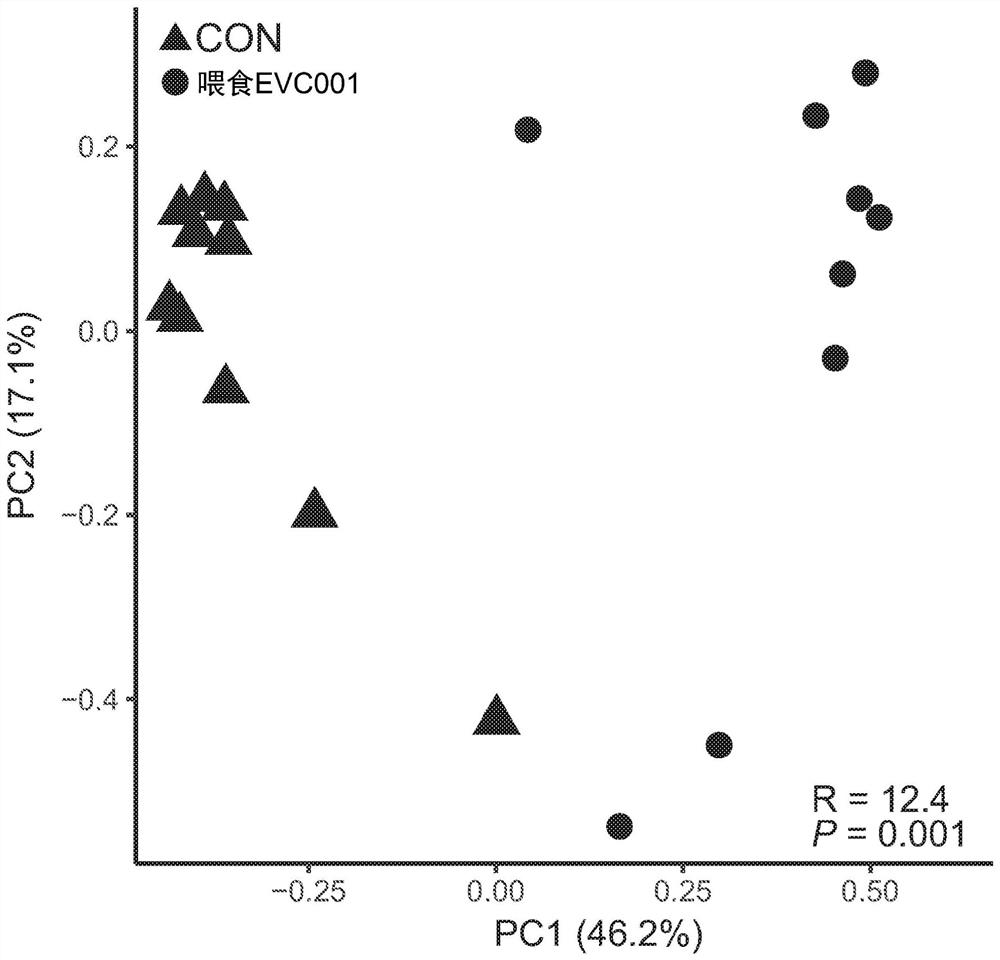
[0159] Feeding Bifidobacterium infantis EVC001 to infants consuming an HMO-enriched diet
[0160] The trial aimed to show the effect of probiotic supplementation with B. longum subsp. infantis (B. infantis EVC001 ) compared to a non-supplemented group in healthy term nursing infants. Purified isolates (strain EVC001, ATCC Accession No. PTA-125180, Evolve Biosystems Inc., Davie, CA) were grown by culturing in the presence of BMO according to International Patent Application No. PCT / US2015 / 057226. Adams, isolated from human infant fecal samples) began with the preparation of dry compositions of lactose and activated Bifidobacterium longum subsp. infantis. The culture was harvested by centrifugation, lyophilized, and the concentrated powder preparation had an activity of approximately 300 billion CFU / g. The concentrated powder is then diluted to an activity level of approximately 30 billion CFU / g by mixing with infant formula grade lactose. The composition is then filled into...
Embodiment 1A
[0167] Metabolomic analysis of infant feces
[0168] Fecal samples from the infants of Example 1 were evaluated as described below to characterize the fecal metabolome and the possible impact of colonization with this organism on the overall metabolism of the infant.
[0169] Sample Preparation: Fecal samples were kept at -80°C until processing. Using automation from HamiltonCompany The system prepares the sample. For quality control purposes, several recovery criteria are added before the first step of the extraction process. To remove proteins, small molecules bound to proteins are dissociated or retained in the precipitated protein matrix and chemically diverse metabolites are recovered by precipitating proteins with methanol for 2 min with vigorous shaking (Glen Mills GenoGrinder 2000), followed by centrifugation . The resulting extract was divided into five fractions: two fractions were analyzed by two independent reversed-phase (RP) / UPLC-MS / MS methods using positiv...
Embodiment 1B
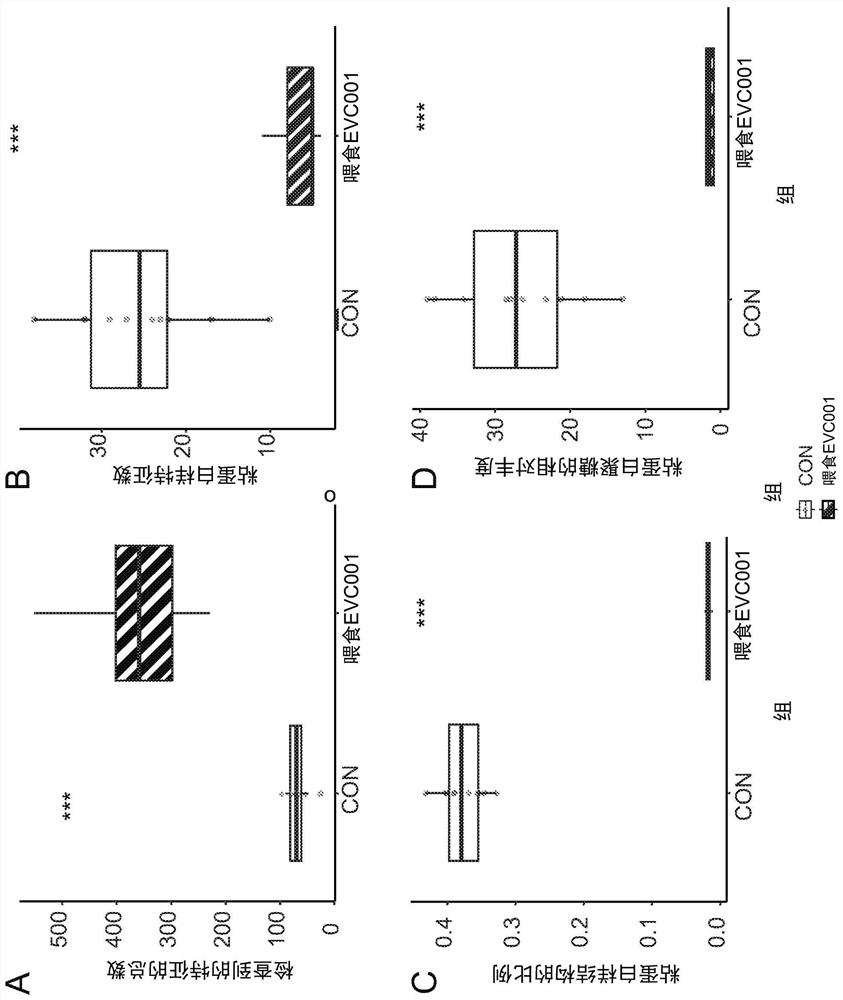
[0191] Increased mucin production
[0192] In this example, mucin degradation was significantly reduced in infants supplemented with EVC001 compared to controls. The following mucin structures were monitored as part of the metabolome in infant feces.
[0193] Table 5 - Changes in mucin structure
[0194]
[0195] A library of known mucin-specific O-glycans was compiled and used to interrogate untargeted mass spectra of stool samples. It was hypothesized that modification of the gut microbiome would lead to modulation of mucin degradation by gut microbes. The second part of the hypothesis is that by using the colonization of Bifidobacterium infantis (which does not degrade mucins) and the subsequent reduction of mucolytic taxa will reduce the degradation of mucins, through the mucin-specific O in infant feces. - Glycan abundance is measured.
[0196] Analysis of spectra obtained with nano-high performance liquid chromatography-chip / time-of-flight mass spectrometry (n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com