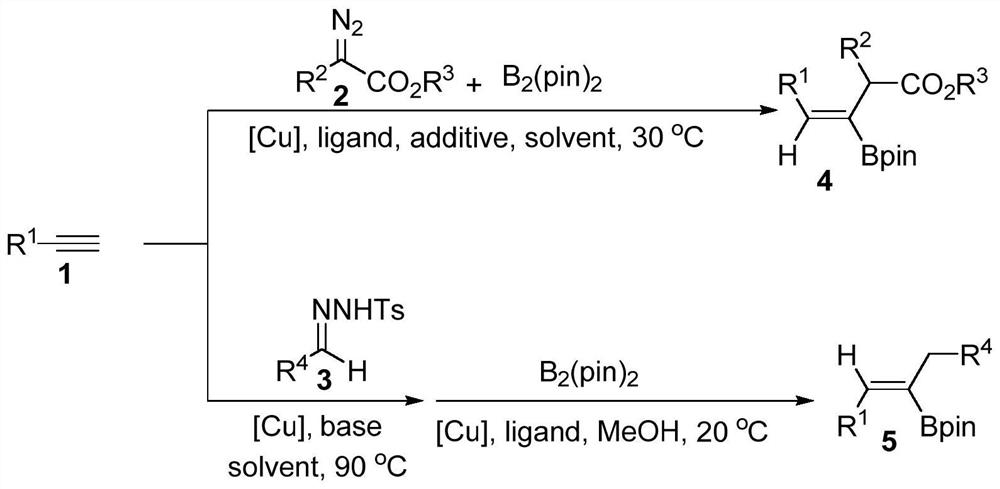
Method for synthesizing trisubstituted alkenyl borate through copper-catalyzed three-component reaction
A technology of alkenyl borate and tri-substitution, which is applied in the field of synthesizing alkenyl borate, can solve the problems that the synthesis of tri-substituted alkenyl borate has not yet been realized, and achieve wide application range of substrates, simple reaction operation, The effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: Synthesis of 4a:
[0032]
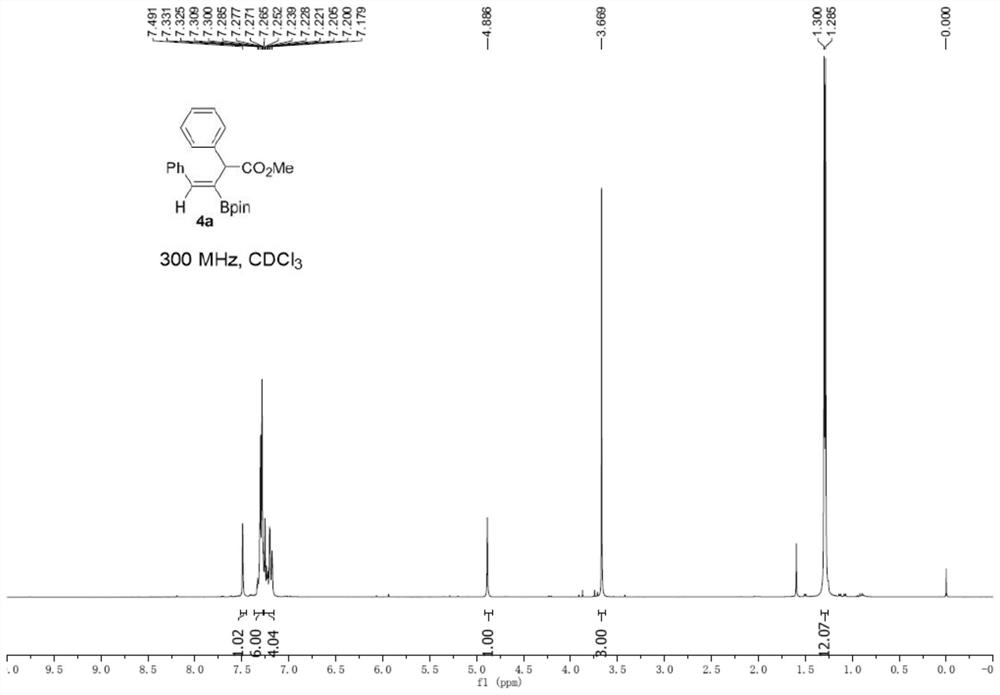
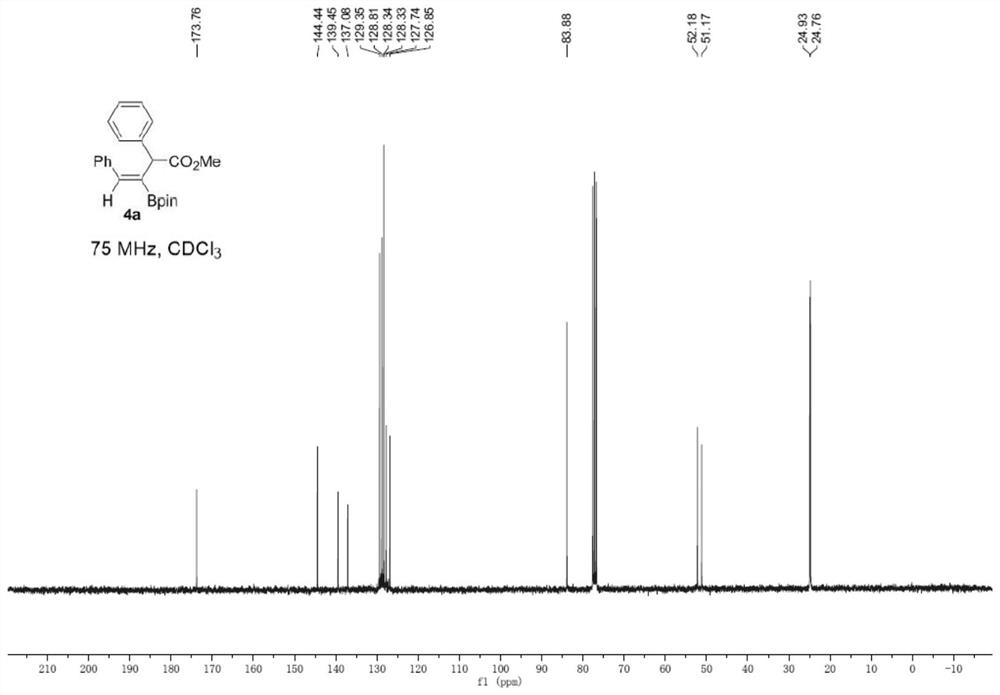
[0033] Under argon protection, add 1a (20.4 mg), B 2 (pin) 2 (152.3mg), CuI (3.1mg), 2,2'-bipyridine (3.1mg), Molecular sieves (100 mg) and DMF (2 mL) were added after which 2a (52.8 mg) dissolved in DMF (2 mL) was added; the reaction mixture was stirred on a 30°C heating block for 18 hours. The reaction solution was diluted with 10 mL of water and extracted with ethyl acetate; the organic phase was dried and the solvent was removed under reduced pressure; the crude product was purified by column chromatography and washed with ethyl acetate:petroleum ether (1:20) to obtain a colorless oily product 4a (66.6 mg, 88%, Z:E>19:1). 1 H NMR (300MHz, CDCl3) δ7.49(s,1H),7.33-7.26(m,6H),7.26-7.17(m,4H),4.89(s,1H),3.67(s,3H),1.30( s,6H), 1.29(s,6H). 13C NMR (75MHz, CDCl3) δ173.8, 144.4, 139.5, 137.1, 129.4, 128.8, 128.34, 128.3, 127.7, 126.9, 83.9, 52.2, 51.2, 24.9, 24.8. HRMS (ESI) m / z: [M+Na] +Calcd for C 23 h 27 BNaO 4 401...
Embodiment 2
[0034] Embodiment 2: Synthesis of 4a:
[0035]
[0036] Under argon protection, add 1a (20.4 mg), B 2 (pin) 2 (152.3mg), CuI (3.1mg), 2,2'-bipyridine (3.1mg), Molecular sieves (100 mg) and DMSO (2 mL) were added followed by 2a (52.8 mg) dissolved in DMSO (2 mL); the reaction mixture was stirred on a 30°C heating block for 18 hours. The reaction solution was diluted with 10 mL of water and extracted with ethyl acetate; the organic phase was dried and the solvent was removed under reduced pressure; the crude product was purified by column chromatography and washed with ethyl acetate:petroleum ether (1:20) to obtain a colorless oily product 4a (30.2 mg, 40%, Z:E>19:1).
Embodiment 3
[0037] Embodiment 3: Synthesis of 4a:
[0038]
[0039] Under argon protection, add 1a (20.4 mg), B 2 (pin) 2 (101.5mg), CuI (3.1mg), 2,2'-bipyridine (3.1mg), Molecular sieves (100 mg) and DMF (2 mL) were added after which 2a (52.8 mg) dissolved in DMF (2 mL) was added; the reaction mixture was stirred on a 30°C heating block for 18 hours. The reaction solution was diluted with 10 mL of water and extracted with ethyl acetate; the organic phase was dried and the solvent was removed under reduced pressure; the crude product was purified by column chromatography and washed with ethyl acetate:petroleum ether (1:20) to obtain a colorless oily product 4a (38.6 mg, 51%, Z:E>19:1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com