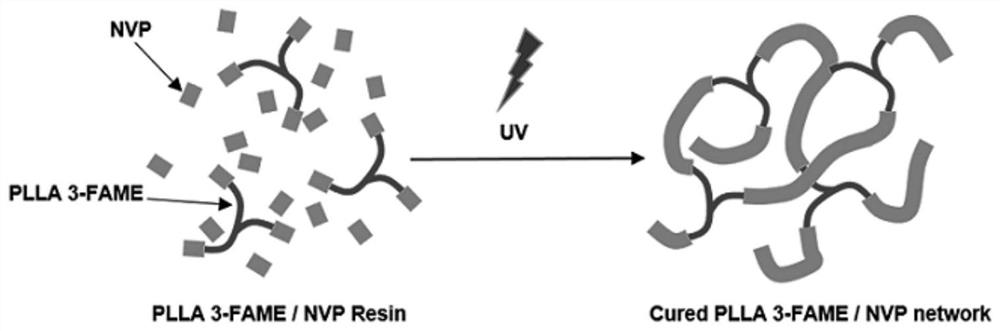
Photosensitive bioabsorbable polymer with in-situ cell adhesion resistance function and preparation method ofphotosensitive bioabsorbable polymer
A technology of absorbing polymers and photosensitivity, which is applied in the field of biomedical polymer material technology and photocuring, which can solve the problems of easy peeling off of the coating and failure of coating modification, and achieve fast curing speed, low cost, and good bioabsorption sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] This embodiment 1 provides a photosensitive bioabsorbable polymer with in situ anti-cell adhesion function, and the specific formula (100 grams) is as follows:
[0034] FAME-modified three-arm photosensitive lactic acid oligomer (FAME-3arms-LAO) 45g
[0035] NVP 55g
[0036] Irgacure 2959 5g
[0037]Wherein, the three-arm type photosensitive lactic acid oligomer (three-arm lactic acid oligomer with double bond) modified by FAME (monoethyl fumarate) is prepared by the following method:
[0038] (1) Add 100g of ethyl acetate and 80g of L-lactide in turn to a round-bottomed flask, and continue to reflux and stir at 75°C until the L-lactide is completely dissolved; then the resulting solution is filtered under reduced pressure and the filtrate is collected; the filtrate Ice bath for more than 5 hours until the needle-like L-lactide crystals are completely precipitated; filter under reduced pressure again, and vacuum-dry to obtain purified L-lactide crystals, and store the...
Embodiment 2
[0046] Example 2 provides a photosensitive bioabsorbable polymer with in situ anti-cell adhesion function, the specific formula (100 grams) is as follows:
[0047] MC-modified three-arm photosensitive lactic acid oligomer (MC-3arms-LAO) 60g
[0048] NVP 32g
[0049] Irgacure 2959 8g
[0050] Wherein, the three-arm type photosensitive lactic acid oligomer (three-arm lactic acid oligomer with double bond) modified by FAME (monoethyl fumarate) is prepared by the following method:
[0051] Wherein, MC (methacryloyl chloride) modified three-arm type photosensitive lactic acid oligomer (three-arm type lactic acid oligomer with double bond) is lower than the three-arm type photosensitive lactic acid oligomer modified by FAME in Example 1. The only difference in the polymer preparation method is that the modifying agent in step (3) is replaced by methacryloyl chloride, and the remaining steps and parameters are the same.
[0052] The preparation method of the above-mentioned photos...
Embodiment 3
[0056] This embodiment 3 provides a photosensitive bioabsorbable polymer with in situ anti-cell adhesion function, and the specific formula (100 grams) is as follows:
[0057] AC-modified three-arm photosensitive lactic acid oligomer (AC-3arms-LAO) 55g
[0058] NVP 44g
[0059] Irgacure 2959 6g
[0060] Wherein, AC (acryloyl chloride) modified three-arm photosensitive lactic acid oligomer (three-arm lactic acid oligomer with double bond) and FAME modified three-arm photosensitive lactic acid oligomer in Example 1 The only difference in the preparation method is that the modifying agent in step (3) is replaced by acryloyl chloride, and the rest of the steps and parameters are the same.
[0061] (1) Add 150g of ethyl acetate and 100g of D-lactide successively in a round bottom flask, and continuously reflux and stir at 75°C until the L-lactide is completely dissolved; then the resulting solution is filtered under reduced pressure and the filtrate is collected; The filtrate wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com