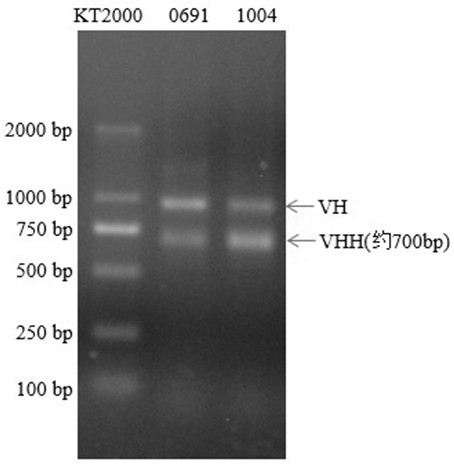
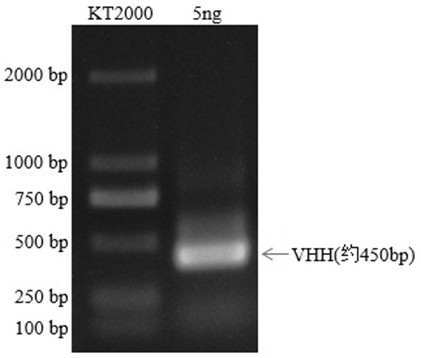
Mcherry or mEOS nano antibody as well as preparation method and application of mcherry or mEOS nano antibody
A nano-antibody and antibody technology, applied in the field of bioengineering, can solve problems such as poor batch stability, poor acid-base stability, and variability, and achieve the effects of low cost, good stability, and strong affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The preparation method of mcherry nanobody comprises the following steps:
[0053] (1) Construction of mcherry nanobody library:
[0054] (1.1) The protein used for immunization is the mcherry protein expressed by Escherichia coli. For the first immunization, take 1 mg of antigenic protein (including 0.25 mg of mcherry protein) and an equal volume of complete Freund's adjuvant, shake, mix and emulsify, and place on the neck of the alpaca Partial point injection, a total of four immunizations, once every two weeks, the amount of each immunization protein is 0.5mg (including 0.125mgmcherry protein), and the adjuvant used for booster immunization is Freund's incomplete adjuvant. About four days after the fourth immunization, collect at least 20mL alpaca whole blood, and use lymphocyte separation medium to separate PBMC.
[0055] (1.2) Extract the total RNA of PBMC (using the trizol method).
[0056] (1.3) RNA was reverse-transcribed into cDNA, and the operation steps wer...
Embodiment 2
[0093] The preparation method of mEOS nanobody comprises the following steps:
[0094] (1) Construction of mEOS nanobody library:
[0095] (1.1) The protein used for immunization is the mEOS protein expressed by Escherichia coli. For the first immunization, take 0.5mg of mEOS protein and an equal volume of Freund's complete adjuvant, shake, mix and emulsify, and inject it at the neck of the alpaca. Four times, one immunization every two weeks, each immunization protein dosage is 0.5 mg, and the adjuvant used for booster immunization is Freund's incomplete adjuvant. About four days after the fourth immunization, collect at least 20mL alpaca whole blood, and use lymphocyte separation medium to separate PBMC.
[0096] (1.2) Extract the total RNA of PBMC (using the trizol method).
[0097] (1.3) RNA was reverse-transcribed into cDNA, and the operation steps were carried out according to the instructions of PrimeScript™ II 1st Strand cDNASynthesis Kit (purchased from takara).
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com