Engineered binding proteins of EpCAM and uses thereof
A technology of binding protein, pet22b-aep3d4-aep4g2, applied in the field of binding protein, can solve the problems of restricting tumor tissue penetration, affecting treatment effect, low immunogenicity, etc., achieving low immunogenicity, high specificity and immunogenicity , the effect of strong tissue penetration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1 obtains negative control binding protein
[0047] The previous experiments in our laboratory used phage display technology to use synthetic peptides of human epidermal growth factor receptor 2 (Her2) and vascular endothelial growth factor (VEGF) as antigens. The above method was used to screen the phage library of human binding proteins, and two were selected by ELISA. For the clones that do not bind to the corresponding antigen, the negative control binding proteins pET22b-aHER2-13C1 and pET22b-aVE201 were respectively obtained. Negative control binding proteins pET22b-aHER2-13C1 and pET22b-aVE201 have the amino acid sequences shown in SEQ ID NO.7 and SEQ ID NO.8 respectively; the nucleotide sequences encoding pET22b-aHER2-13C1 and pET22b-aVE201 are shown in SEQ ID NO .15 and SEQ ID NO.16.
Embodiment 2
[0048] The construction of the binding protein particle of embodiment 2 transformation
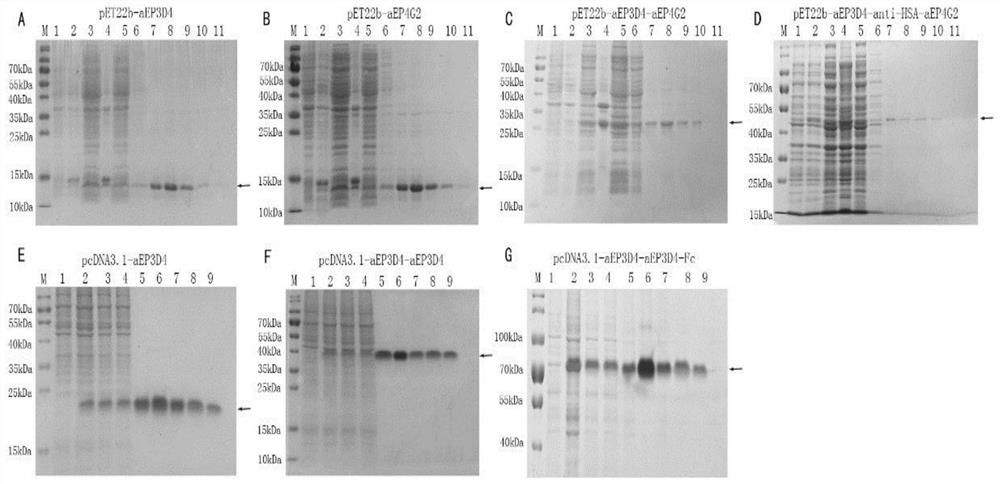
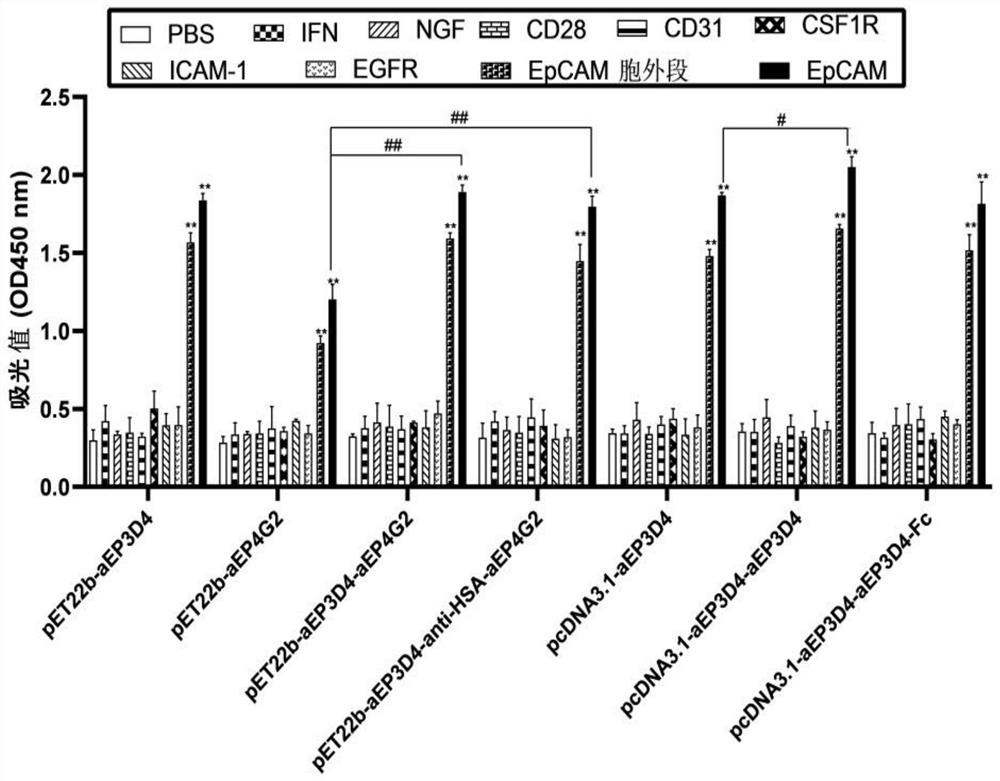
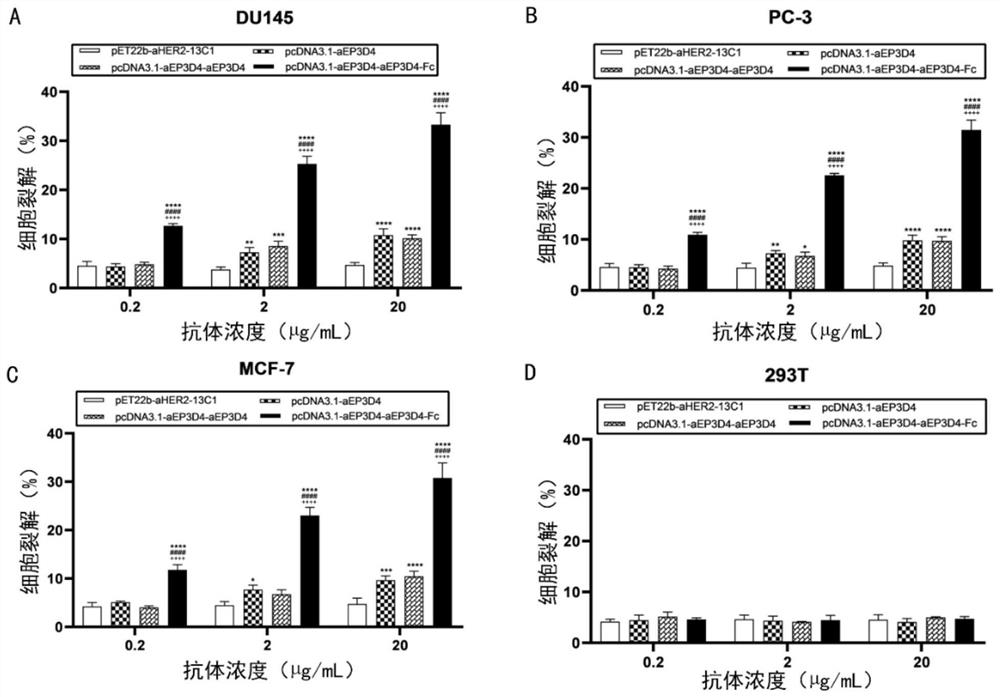
[0049] In the early stage, our laboratory screened out 2 fully human EpCAM binding proteins pET22b-aEP3D4 and pET22b-aEP4G2 by screening phage library, and obtained their nucleotide sequences by gene sequencing. This example uses (G 4 S) 3 The linker connects the nucleotide sequences of the above two human EpCAM binding proteins, and submits it to Jinweizhi Company to synthesize the nucleotide sequence of the pET22b-aEP3D4-aEP4G2 modified binding protein; by using (G 4 S) 3 The linker connects the nucleotide sequence of the above two human EpCAM binding proteins with the nucleotide sequence of the anti-HSA protein, and sends it to Jinweizhi Company to synthesize the nucleoside of the binding protein modified by pET22b-aEP3D4-anti-HSA-aEP4G2 acid sequence. And the above two nucleotide sequences were connected to the prokaryotic expression plasmid pET22b(+) by enzyme digestion to construc...
Embodiment 3
[0050] Prokaryotic expression of the binding protein of embodiment 3 transformation
[0051] 1. Extraction of plasmid: according to the laboratory purchased Operate with the enclosed manual of the Plasmid Mini Kit.
[0052] 2. Transform the prokaryotic expression plasmid into BL21(DE3)
[0053] (1) Take out the prepared BL21(DE3) competent cells from the -80°C ultra-low temperature refrigerator, and put them on ice to thaw.
[0054] (2) After the competent cells are thawed, add 0.1 μg of the prokaryotic expression plasmid constructed above, flick the EP tube with your fingers to mix well, and put it on ice again for half an hour.
[0055] (3) Place in a 42°C water bath, heat shock for 2 minutes, and then quickly place on ice for 2 minutes.
[0056] (4) Add 900 μL of autoclaved LB medium, place in a constant temperature shaker at 37°C, shake the bacteria for 1 hour, then centrifuge, discard 900 μL of the supernatant and mix the bacterial pellet.
[0057] (5) Using a steril...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com