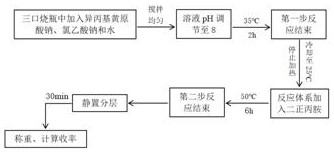
Synthesis and purification method of O-isopropyl-N, N '-di-n-propyl thiocarbamate
A technology of propyl thiocarbamate and synthesis method, which is applied in the field of collectors, can solve the problems of poor selectivity, difficult synthesis, and low purity, and achieve simple operation, high product yield, and improved collection capacity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
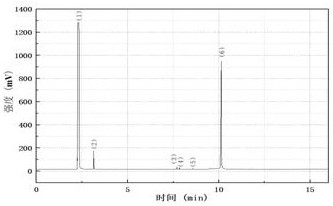
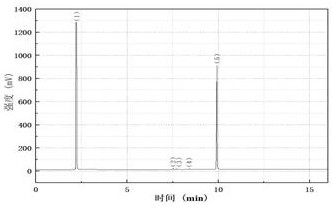
[0045] Combine below Figure 1 to Figure 5 , the present invention is further described:
[0046] Part I, Experimental Reagents and Instruments
[0047] The main experimental reagents used in the present invention are shown in Table 1-1. Among them, isopropanol, carbon disulfide and sodium hydroxide are used to synthesize sodium isopropyl xanthate.
[0048] Table 1-1 Main reagents for experiment
[0049] name chemical formula molecular weight Specification Manufacturer Isopropanol C 3 h 7 Oh
60.06 Analytical pure Tianjin Damao Chemical Reagent Factory carbon disulfide CS 2
76.14 Analytical pure Sinopharm Chemical Reagent Co., Ltd. sodium hydroxide NaOH 40.00 Analytical pure Xilong Chemical Co., Ltd. Sodium Chloroacetate ClCH 2 COONa
116.50 Analytical pure Tianjin Guangfu Fine Chemical Research Institute Di-n-propylamine (C 3 h 7 ) 2 NH
101.19 Analytical pure Shanghai Hansi Che...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com