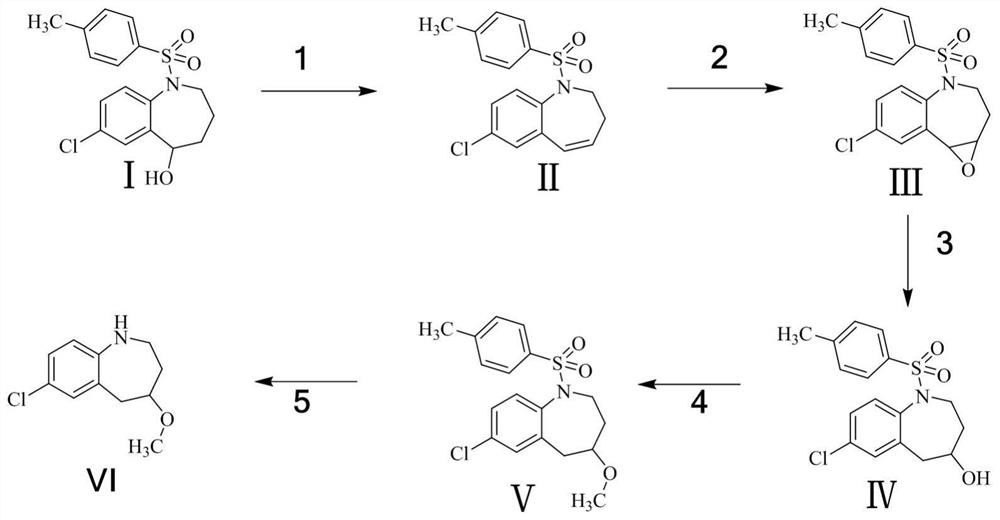
Synthesis of a Degraded Derivative of Tolvaptan
A technology for tolvaptan and derivatives is applied in the field of synthesis of tolvaptan degradation derivatives, and achieves the effects of simple experimental operation, reasonable route design, and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] as Figure 1 As shown, a method of synthesis of tolvaptan degraded derivatives, comprising the following steps:
[0032] Step (1): Preparation of Compound II
[0033] Take 3.0g of raw material I. dissolved in 30mL of toluene, and then add 1.67g of concentrated sulfuric acid, 110 °C reaction, TLC monitoring reaction end. The reaction solution was washed with saturated sodium bicarbonate aqueous solution, and then directly concentrated and steamed dry, and column chromatography was purified to give 2.5g of compound II., with a yield of 87.8%.
[0034]
[0035] Step (2): Preparation of Compound III
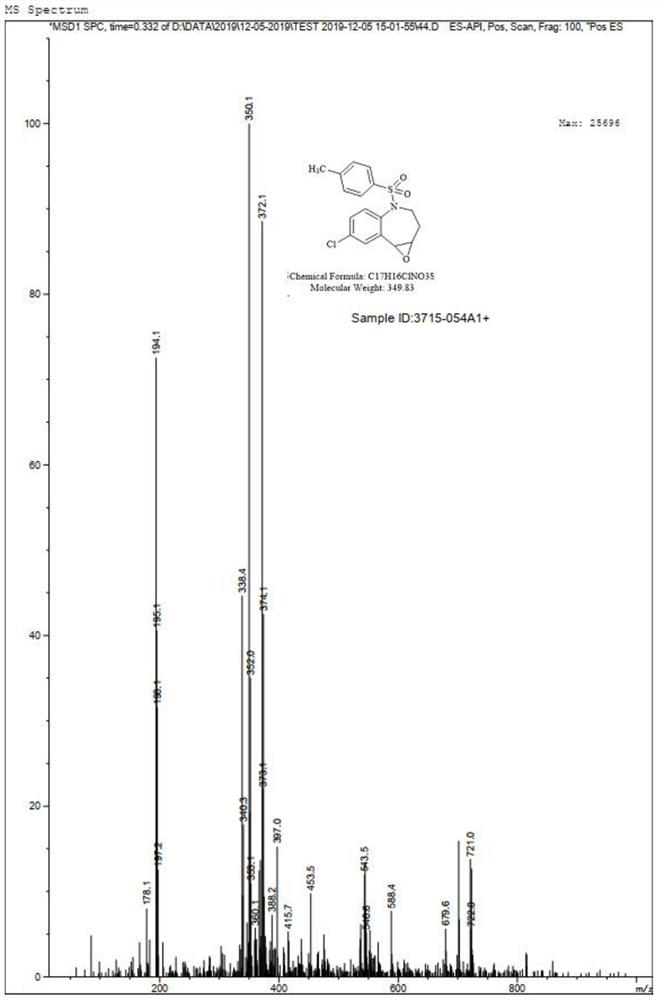
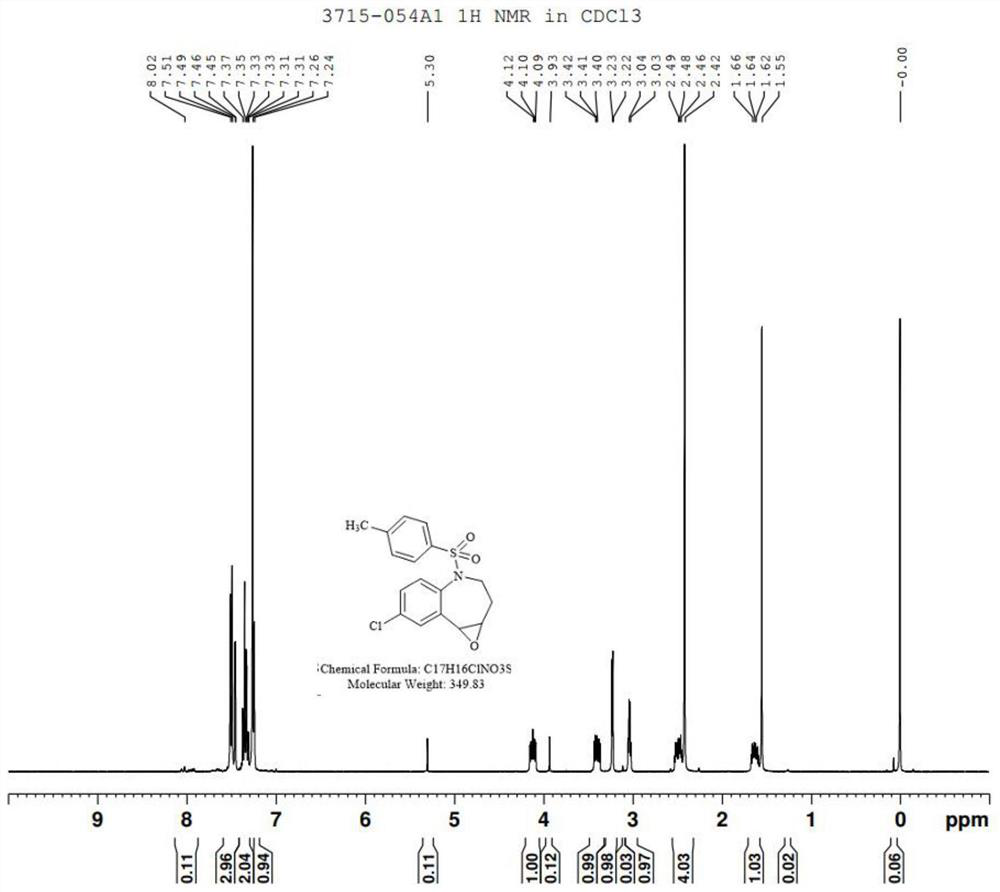
[0036] Take 2.5g of compound II. dissolved in 50mL of methanol, add 1.04g of hydrogen peroxide, after a period of reaction, TLC showed that the reaction was complete. Under the ice bath, sodium sulfite solution was added to quench the remaining oxidant, methanol was concentrated, and the aqueous phase was extracted with ethyl acetate to concentrate 2.40g of compound III., with a ...
Embodiment 2
[0048] as Figure 1 As shown, a method of synthesis of tolvaptan degraded derivatives, comprising the following steps:
[0049] Step (1): Preparation of Compound II
[0050]Take 9.0g of raw material I. dissolved in 90mL of toluene, and then add 5g of concentrated sulfuric acid, 110 °C reaction, TLC monitoring the end of the reaction. The reaction solution was washed with saturated sodium bicarbonate aqueous solution, and then directly concentrated and steamed dry, and column chromatography was purified to obtain 7.47g of compound II., with a yield of 87.5%.
[0051]
[0052] Step (2): Preparation of Compound III
[0053] Take 7.3g of compound II. dissolved in 120mL of methanol, add 2.5g of hydrogen peroxide, after a period of reaction, TLC showed that the reaction was complete. Under the ice bath, sodium sulfite solution was added to quench the remaining oxidant, methanol was concentrated, and ethyl acetate was extracted in the aqueous phase to give 6.98g of compound III., NMR ex...
Embodiment 3
[0065] as Figure 1 As shown, a method of synthesis of tolvaptan degraded derivatives, comprising the following steps:
[0066] Step (1): Preparation of Compound II
[0067] Take 45.0g of raw material I. dissolved in 270mL of toluene, and then add 15.03g of concentrated sulfuric acid, 110 °C reaction, TLC monitoring reaction end. The reaction solution was washed with saturated sodium bicarbonate aqueous solution, and then directly concentrated and steamed dry, and column chromatography was purified to give 37.23g of compound II., with a yield of 87.2%;
[0068]
[0069] Step (2): Preparation of Compound III
[0070] Take 37.0g of compound II. dissolved in 720mL of methanol, add 14.98g of hydrogen peroxide, after a period of reaction, TLC showed that the reaction was complete. Under the ice bath, sodium sulfite solution was added to quench the remaining oxidant, methanol was concentrated, and the aqueous phase was extracted with ethyl acetate, concentrated to give 35.40g of compou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com