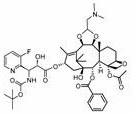
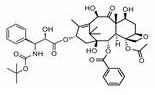
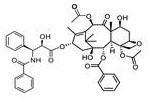
Methods of treating CNS tumors with tesetaxel
A technology of tesostatin and tumor, which is applied in the field of treating CNS tumors with tesostatel, which can solve the problems of bad results and so on.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Example 1: Clinical Research
[0098] Patients with HER2-negative, HR-positive LA / MBC previously treated with taxanes in the neoadjuvant or adjuvant setting were recruited and randomized into one of two treatment groups. Known transfers to CNS are permitted but not required. Additional analyzes of CNS tumor efficacy were performed on patients with CNS tumor metastases.
[0099] Patients in treatment group 1 received 27 mg / m orally on day 1 of a 21-day cycle 2 Tesetaxel, and started on day 1 of a 21-day cycle and ended on day 15, 14 doses of 1,650 mg / m orally 2 The daily dose of capecitabine (825mg / m 2 , twice daily at intervals), beginning with an evening dose on Day 1 of each 21-day cycle and ending with a morning dose on Day 15. Treatment continued in 21-day cycles until a patient's disease progression or unacceptable toxicity was observed.
[0100] Beginning on day 1 of a 21-day cycle and ending on day 15, with 14 doses of 2,500 mg / m 2 Daily dose of capecitab...
Embodiment 2
[0102] Embodiment 2: clinical research
[0103] Elderly patients (≥65 years) with HER2-negative LA / MBC not previously treated with chemotherapy for LA / MBC were assigned to one treatment group without randomization. Inclusion criteria included prior endocrine therapy with or without a CDK 4 / 6 inhibitor, unless endocrine therapy was not specified. Known CNS transfers are allowed but not required.
[0104] On day 1 of each 21-day cycle, use 27 mg / m 2 Tesetaxel monotherapy was administered orally to patients once. Treatment continued in 21-day cycles until documented progressive disease (PD), unacceptable toxicity observed in the patient, or other decision to discontinue treatment.
[0105] The primary endpoint of the study was the investigator-assessed objective response rate using RECIST 1.1 criteria. Secondary endpoints included investigator-assessed progression-free survival and overall survival using RECIST 1.1 criteria. Efficacy on CNS metastasis is measured by CNS ob...
Embodiment 3
[0106] Embodiment 3: clinical research
[0107] Adult patients (≥18 years) with triple-negative LA / MBC who had not received prior chemotherapy for LA or metastatic disease were recruited and randomized into three treatment groups. The patient's most recent biopsy must be negative for HR. Known transfers to CNS are permitted but not required.
[0108] Administer 27 mg / m orally on day 1 of each 21-day cycle to treated patients 2 Tesetaxel, plus one of the following: (A1) Nivolumab (360 mg) IV infusion over 30 minutes on Day 1 of each 21-day cycle; (A2) Nivolumab (360 mg) on Day 1 of each 21-day cycle pembrolizumab (200 mg) intravenously for 30 minutes every day; or (A3) atezolizumab (1200 mg) intravenously for 60 minutes on day 1 of each 21-day cycle (if the first infusion is allowed, all Subsequent infusions can be infused for 30 minutes). Treatment continued in 21-day cycles until documented progressive disease, unacceptable toxicity observed in the patient, or other d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com