Application of erianin in preparation of osteoclast differentiation inhibitor
A technology for osteoclast differentiation inhibition, applied in anti-inflammatory agents, bone diseases, non-central analgesics, etc., can solve problems such as lack of osteoclast differentiation inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
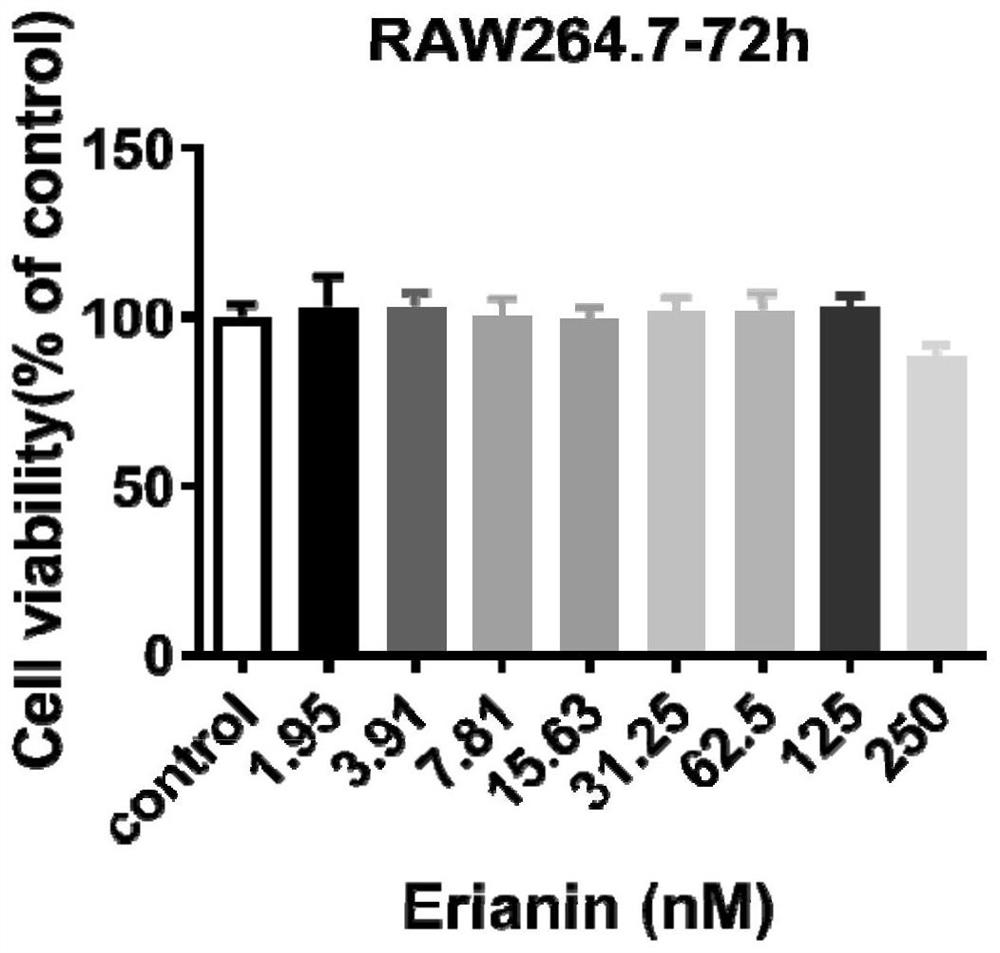
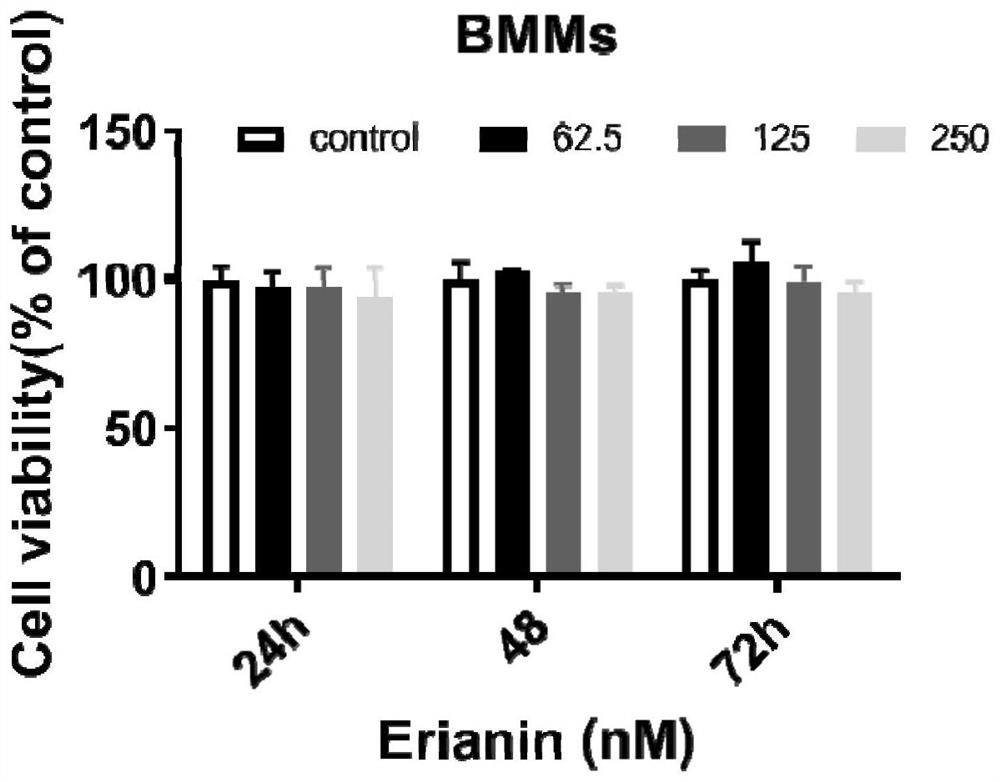
[0041] Experimental example 1. The effect of erianin on the cell survival of RAW264.7 cells and mouse bone marrow macrophages (BMMs):
[0042] RAW264.7 or BMMs cells in good growth state were inoculated in 96-well plates. After overnight attachment, the negative control group (Control group) was not added with Erianin, and the drug group was added with 1.95–250nM Erianin. After culturing in a 5% CO2 incubator for 24h, 48h, and 72h, discard the medium, add 100 μl / well MTT solution (0.5mg / ml), and incubate for 4h. Add 150 μl / well DMSO, mix thoroughly with a micro-oscillator at low speed for 10 minutes, and measure the OD value at 570 nm with a TECAN GENios Pro multifunctional microplate reader. The result is as figure 1 , figure 2 .
[0043] Depend on figure 1 , figure 2 It can be seen that within 72 hours, erianin with an in vitro concentration of ≤250nM has no significant effect on the survival of RAW264.7 cells and BMMCs.
experiment example 2
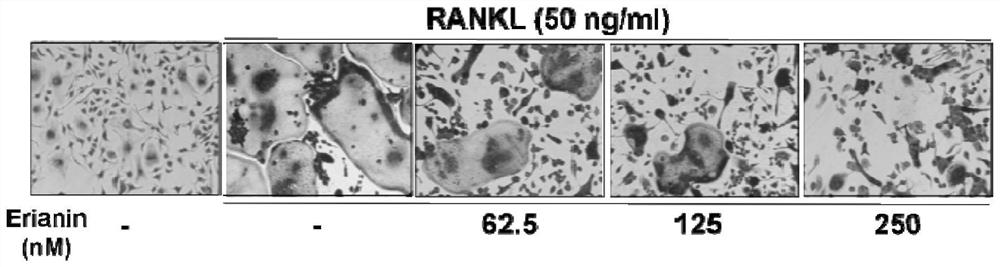
[0044] Experimental Example 2. Effect of Erianin on RANKL-induced differentiation of mouse bone marrow macrophages (BMMs) into osteoclasts:
[0045] BMMs were seeded in 96-well plates, and after adhering to the wall overnight, in addition to the negative control group (Blank group), the positive control group (RANKL group) and the drug group were added with M-CSF and RANKL (the final concentration of M-CSF was 30ng / ml, The final concentration of RANKL was 50ng / ml), and the drug group was simultaneously added with erianin concentrations of 62.5nM, 125nM, and 250nM, and each group had three replicate wells. Change the medium every 2 days. On the 4th day of induction, TRAP staining was observed, photographed under a microscope, and counted as osteoclasts that were positive for TRAP and had more than 3 nuclei (osteoclasts were purplish red and multiple cells fused together). The result is as image 3 , Figure 4 .
[0046] Combined with the results of the cell survival experim...
experiment example 3
[0047] Experimental Example 3. Effect of Erianin on RANKL-induced osteoclast activity:
[0048] Inoculate BMMs cells with the same protocol of osteoclast generation in Experimental Example 2 above and culture them on the Osteo Assay 96-well plate coated with artificial bone slices. On the 7th day of induction, the cells were washed away, observed and photographed with an inverted optical microscope, and photographed through Image- Pro Plus software calculates the percentage of bone resorption area in each well. The result is as Figure 5 , Image 6 .
[0049] The experimental results prove that erianin with concentrations of 62.5nM, 125nM, and 250nM can significantly reduce the area of bone depressions formed by osteoclasts on bone slices, and have an inhibitory effect on the bone resorption activity of osteoclasts.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com