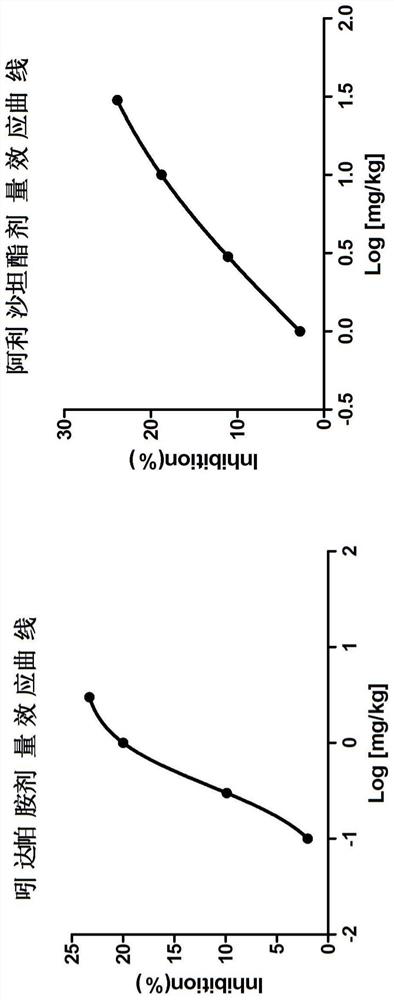
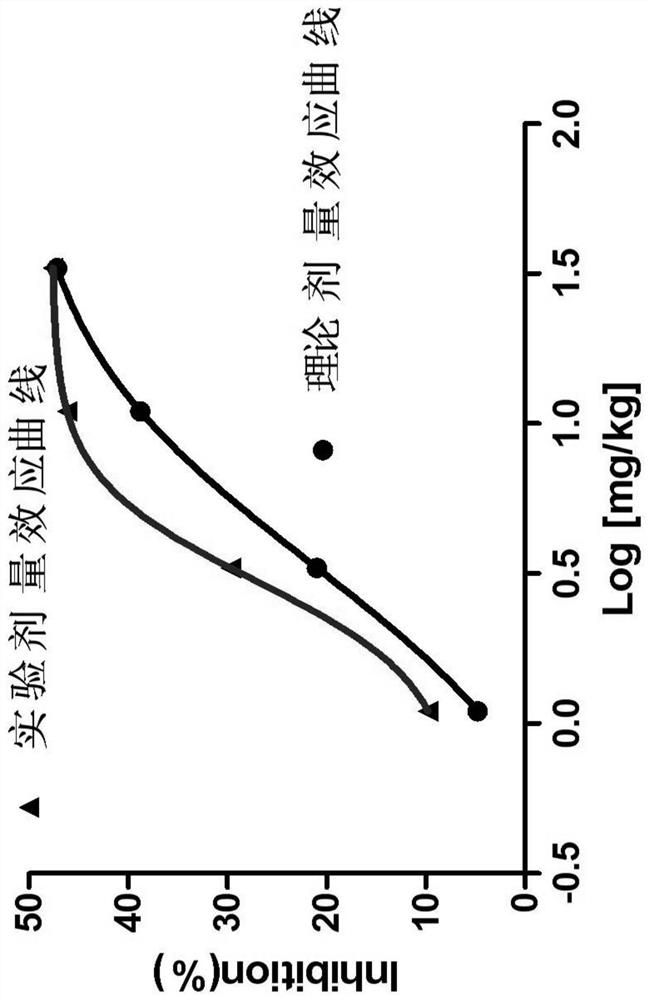
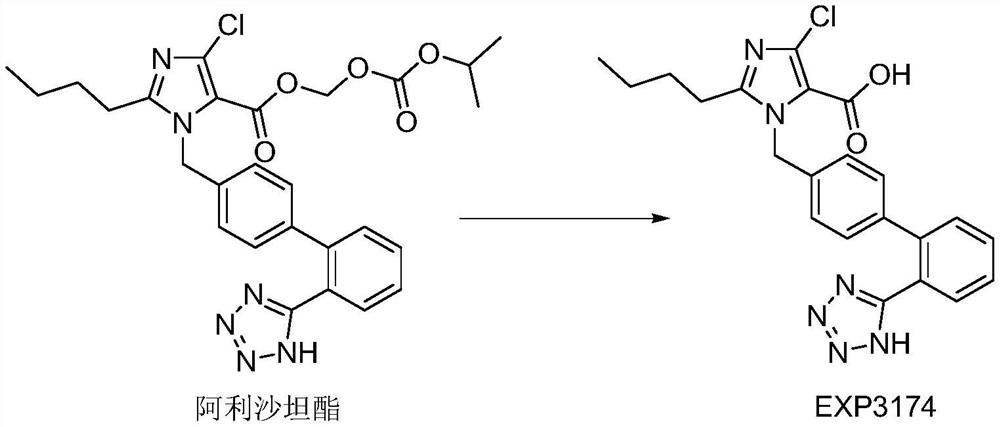
Pharmaceutical composition of allisartan medoxomil or salt thereof and diuretic
A technology of allisartan medoxomil and a composition, applied in the field of medicinal chemistry, can solve the problems such as the inability to achieve the best therapeutic effect, the undisclosed amount of the diuretic agent, the undisclosed amount of the diuretic agent, etc., and achieves reduction of adverse drug reactions and strong operability. , the effect of simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1 prepares alisartan medoxomil-indapamide pharmaceutical composition
[0026] Allisartan medoxomil (4.8 kg) was added in the V-shaped mixing cylinder, and indapamide (0.5 kg) was added, and after mixing for 60 minutes, the alisartan medoxomil-indapamide pharmaceutical composition with a mass ratio of 96:1 was obtained.
[0027] The same method can be used to prepare other alisartan medoxomil-indapamide pharmaceutical compositions with other mass ratios, such as alisartan medoxomil-indapamide pharmaceutical compositions with mass ratios of 48:1 and 300:1.
Embodiment 2
[0028] Embodiment 2 prepares alisartan medoxomil potassium-indapamide pharmaceutical composition
[0029] Allisartan medoxomil potassium (2.4 kg, calculated as allisartan medoxomil) was added in the V-type mixing cylinder, added indapamide (0.5 kg), and after mixing for 30 minutes, added allisartan medoxomil potassium (2.4 kg, calculated as allisartan medoxomil) ), and after further mixing for 30 minutes, it is the alisartan medoxomil potassium-indapamide pharmaceutical composition that the mass ratio is 48:1.
[0030] The same method can be used to prepare the other mass ratios of alisartan medoxomil potassium-indapamide pharmaceutical compositions, such as the alisartan medoxomil potassium-indapamide pharmaceutical compositions with mass ratios of 96:1 and 300:1.
Embodiment 3
[0031] Embodiment 3 prepares other alisartan medoxomil salt-indapamide pharmaceutical composition
[0032] The same method as in Example 1 or 2 can be used to prepare the pharmaceutical composition of other salts of alisartan medoxomil and indapamide, such as other salts of alisartan medoxomil and indapamide in mass ratios of 48:1, 96:1, and 300:1. Pharmaceutical compositions of dapamide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com