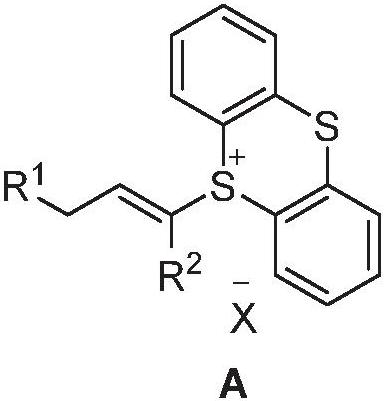
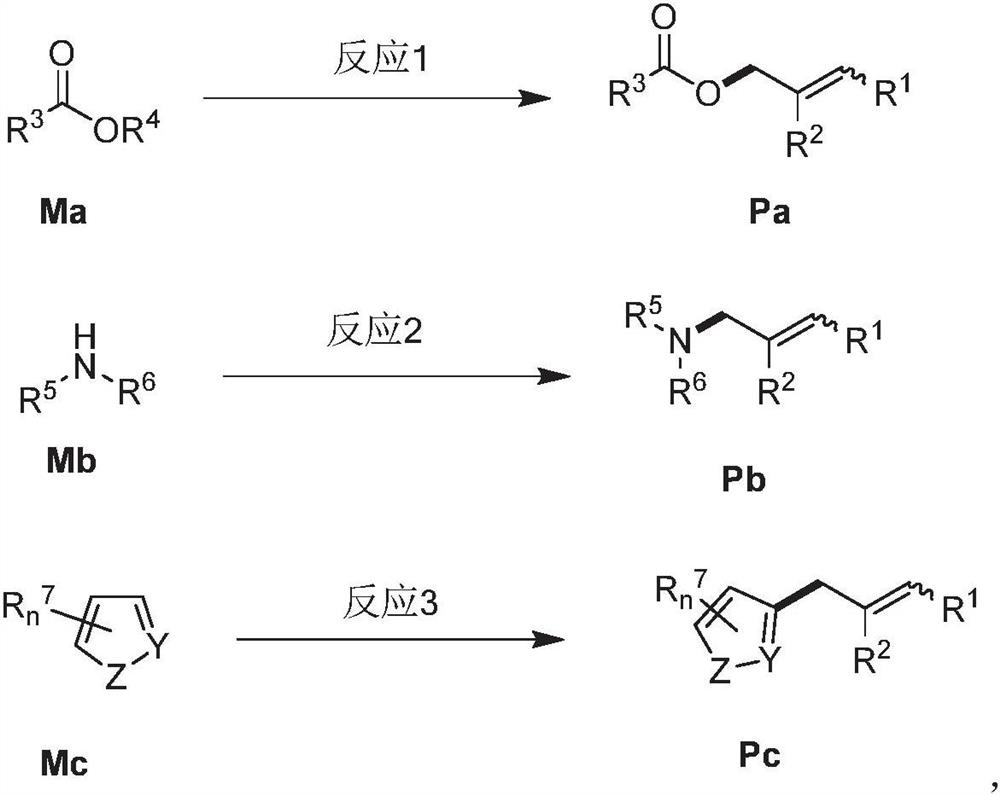
Allylation coupling reaction method and application thereof
A technology of allylation coupling and coupling reaction, which is applied in chemical instruments and methods, condensation/addition reactions to prepare amino compounds, organic chemistry, etc., and can solve problems such as the inability to realize secondary amine allylation reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065]
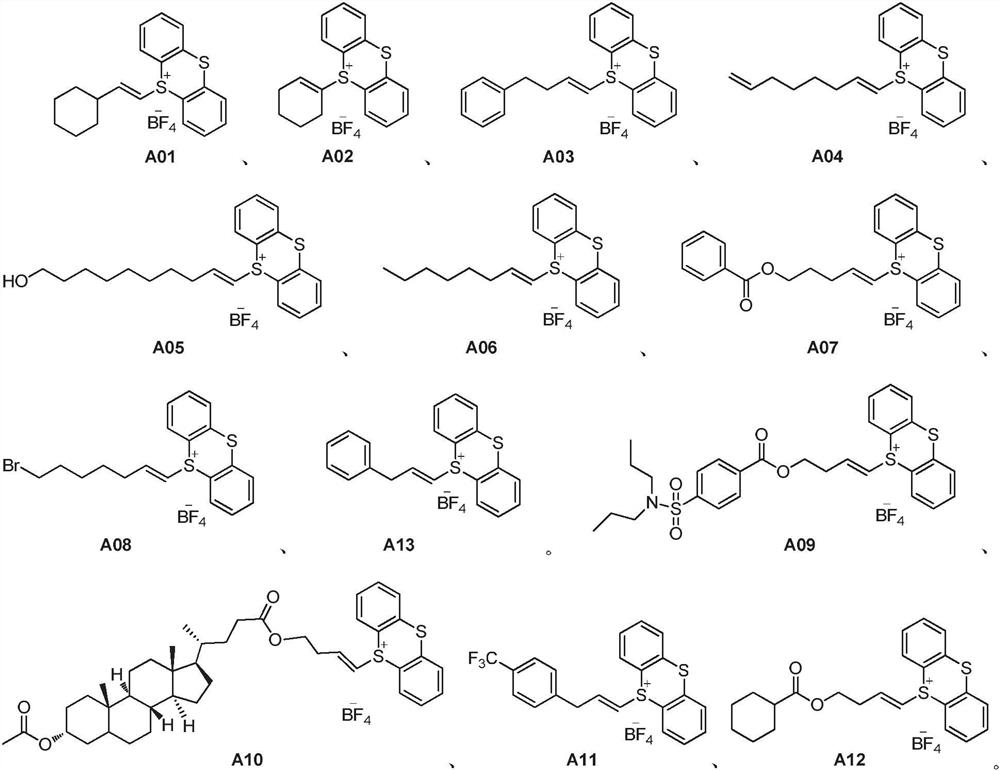
[0066] First weigh compound M01 (29.3mg, 0.24mmol), K 2 CO 3 (27.6mg, 0.20mmol) and compound A01 (82.4mg, 0.20mmol) were added to the reaction tube, and the gas was purged three times through the vacuum line. Under nitrogen atmosphere, DCM (2.0mL) was added and reacted at room temperature for 24h. After the reaction was completed, the solvent was removed by rotary evaporation, and the sample was applied by dry method. Column chromatography (300-400 mesh chromatography silica gel) (petroleum ether-ethyl acetate=19:1) yielded 41.9 mg of product P01 with a yield of 91%.
[0067] 1 H NMR (400MHz, CDCl 3 )δ (ppm): 8.09-8.01 (m, 2H), 7.59-7.50 (m, 1H), 7.43 (dd, J = 8.4, 7.0Hz, 2H), 5.42 (tt, J = 7.2, 1.3Hz, 1H ), 4.84(d, J=7.2Hz, 2H), 2.32-2.24(m, 2H), 2.20-2.12(m, 2H), 1.58(p, J=2.3Hz, 6H);
[0068] 13 C NMR (101MHz, CDCl 3 )δ (ppm): 166.81, 147.21, 132.88, 130.73, 129.72, 128.41, 115.36, 61.28, 37.14, 29.27, 28.50, 27.93, 26.75;
[0069] HRMS-ESI(m / z): 231.1378[M...
Embodiment 2
[0071]
[0072] Weigh K first 2 CO 3 (27.6mg, 0.20mmol) and compound A01 (82.4mg, 0.20mmol) were added to the reaction tube, and the gas was purged three times through the vacuum line. Under nitrogen atmosphere, DCM (2.0mL) and compound M02 (14.4mg, 0.24mmol) were added , Reaction at room temperature for 24h. After the reaction was completed, the solvent was removed by rotary evaporation, and the sample was applied by dry method. Column chromatography (300-400 mesh chromatography silica gel) (petroleum ether-ethyl acetate=19:1) yielded 21.6 mg of product P02 with a yield of 64%.
[0073] 1 H NMR (400MHz, CDCl 3 )δ(ppm): 5.28(tt, J=7.3, 1.2Hz, 1H), 4.58(d, J=7.3Hz, 2H), 2.22-2.17(m, 2H), 2.11(d, J=5.6Hz, 2H), 2.05(s, 3H), 1.56(tt, J=6.9, 4.5Hz, 6H);
[0074] 13 C NMR (100MHz, CDCl 3 )δ (ppm): 171.29, 147.07, 115.26, 60.81, 37.14, 29.14, 28.46, 27.86, 26.74, 21.25;
[0075] HRMS-ESI(m / z): 169.1225[M+H] + .
Embodiment 3
[0077]
[0078] Weigh K first 2 CO 3 (27.6mg, 0.20mmol) and compound A01 (82.4mg, 0.20mmol) were added to the reaction tube, and the gas was purged three times through the vacuum line. Under nitrogen atmosphere, DCM (2.0mL) and compound M03 (11.0mg, 0.24mmol) were added , Reaction at room temperature for 24h. After the reaction was completed, the solvent was removed by rotary evaporation, and the sample was applied by dry method. Column chromatography (300-400 mesh chromatography silica gel) (petroleum ether-ethyl acetate=19:1) yielded 22.8 mg of product P03 with a yield of 74%.
[0079] 1 H NMR (400MHz, CDCl 3 )δ(ppm): 8.06(s, 1H), 5.31(tt, J=7.4, 1.3Hz, 1H), 4.68(d, J=7.4Hz, 2H), 2.21(t, J=5.5Hz, 2H) , 2.16-2.09(m, 2H), 1.56(h, J=4.2Hz, 7H);
[0080] 13 C NMR (100MHz, CDCl 3 )δ (ppm): 161.26, 147.99, 114.64, 60.17, 37.12, 29.15, 28.44, 27.84, 26.69;
[0081] HRMS-ESI(m / z): 155.1066[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com