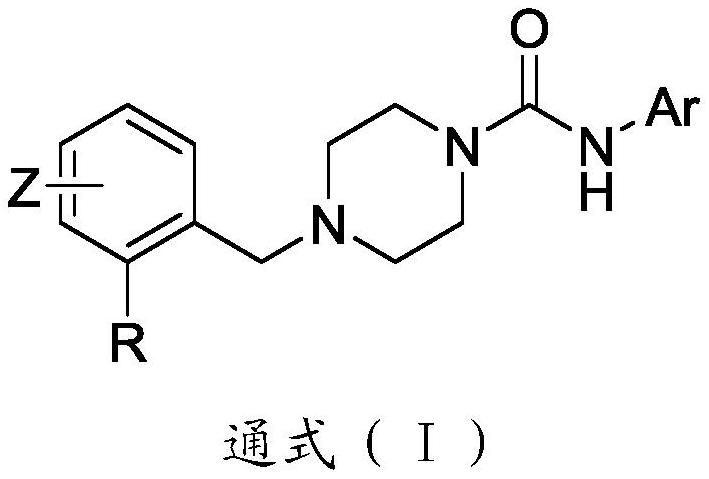
Benzylpiperazine urea TRPV1 antagonistic and MOR agonistic double-target drug as well as preparation method and application thereof
A benzylpiperazine, dual-target technology, used in drug combinations, pharmaceutical formulations, antipyretics, etc., can solve problems such as nausea and vomiting, addiction, adverse reactions, etc., to reduce side effects and have broad analgesic applications Effects of Prospects and Practical Values
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
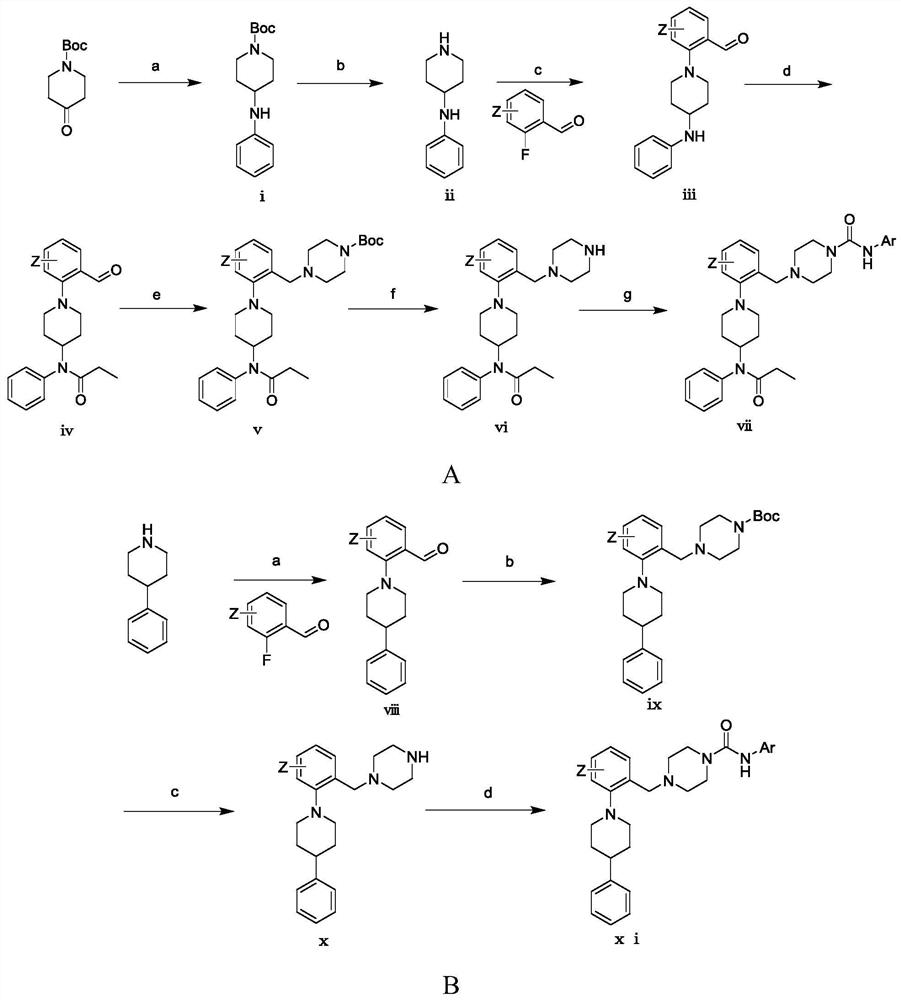
[0047] Example 1: N-(4-(tert-butyl)phenyl)-4-(2-(4-(N-phenylpropionamido)piperidin-1-yl)benzyl)piperazine-1- Preparation of formamide (compound (1))
[0048]
[0049] The preparation method comprises the following steps:
[0050] (a) Preparation of 4-(phenylamino)piperidine-1-carboxylic acid tert-butyl ester
[0051] Dissolve 4.582mL (0.0502mol) of aniline in 200mL of dichloromethane, add 10g (0.0502mol) of 1-Boc-4-piperidone, 2.870mL (0.0502mol) of glacial acetic acid, sodium triacetoxyborohydride 31.911g (0.151mol), stirred at room temperature for 14h, added 100mL of saturated aqueous sodium bicarbonate solution to quench the reaction, extracted 3 times with dichloromethane, 100mL each time, dried over anhydrous sodium sulfate, concentrated under reduced pressure to give 4-(phenylamino ) tert-butyl piperidine-1-carboxylate is dark yellow solid.
[0052] (b) Preparation of N-phenylpiperidin-4-amine
[0053] Dissolve 11.322 g (0.0409 mol) of tert-butyl 4-(phenylamino)pi...
Embodiment 2
[0065] Example 2: N-(3-isopropylphenyl)-4-(2-(4-(N-phenylpropanylamino)piperidin-1-yl)benzyl)piperazine-1-carboxamide (Compound (2)) preparation
[0066]
[0067] Replace the 4-tert-butyl-aniline in step (g) of Example 1 with 104 μL (0.737 mmol) of 3-isopropyl-aniline, and refer to the preparation method in Example 1 for other steps to obtain compound (2) , to obtain a white solid, yield: 63%. The experimental data are as follows:
[0068] C 35 h 45 N 5 o 2 ;yield: 63%, mp=142.9-143.6°C; 1 H NMR (400MHz, CDCl 3 )δppm 7.50-7.31(m, 4H, Ar-H), 7.29-6.99(m, 8H, Ar-H), 6.90(d, J=7.3Hz, 1H, Ar-H), 6.42(d, J= 8.2Hz, 1H, NH), 4.75(ddd, J=12.1, 8.4, 3.9Hz, 1H, Piperidine), 3.41(dd, J=12.9, 8.2Hz, 6H, Ar-CH 2 ,Piperazine), 3.12(d,J=11.7Hz,2H,Piperidine),2.93-2.74(m,3H,CH,Piperidine),2.49-2.29(m,4H,Piperazine),1.94(q,J=7.4Hz ,2H,CH 2 ), 1.86 (d, J = 10.2Hz, 2H, Piperidine), 1.49 (qd, J = 11.9, 3.5Hz, 2H, Piperidine), 1.25 (dd, J = 12.4, 7.0Hz, 6H, CH 3 ), 1.03(t, J=7.4Hz, ...
Embodiment 3
[0069] Example 3: N-(2-methoxyphenyl)-4-(2-(4-(N-phenylpropionamido)piperidin-1-yl)benzyl)piperazine-1-carboxamide (Compound (3)) preparation
[0070]
[0071] Replace the 4-tert-butyl-aniline in step (g) of Example 1 with 83 μL (0.737 mmol) of 2-methoxy-aniline, and refer to the preparation method in Example 1 for other steps to obtain compound (3) , to obtain a white solid, yield: 61%. The experimental data are as follows:
[0072] C 33 h 41 N 5 o 3;yield: 61%, mp=161.9-163.3°C; 1 H NMR (400MHz, CDCl 3 )δppm 8.18-8.12 (m, 1H, NH), 7.44 (tdd, J = 6.8, 4.6, 2.2Hz, 3H, Ar-H), 7.40-7.33 (m, 1H, Ar-H), 7.25-7.17 ( m,1H,Ar-H),7.17-7.10(m,2H,Ar-H),7.11-6.99(m,3H,Ar-H),6.99-6.90(m,2H,Ar-H),6.85( dt,J=4.2,3.5Hz,1H,Ar-H),4.76(tt,J=12.0,3.7Hz,1H,Piperidine),3.86(s,3H,Ar-OCH 3 ), 3.43 (dd, J=11.9, 7.1Hz, 6H, Ar-CH 2 ,Piperazine), 3.13(d,J=11.8Hz,2H,Piperidine),2.82(t,J=11.2Hz,2H,Piperidine),2.48-2.33(m,4H,Piperazine),1.95(q,J=7.4 Hz, 2H, CH 2 ), 1.87(d, J=10.0Hz, 2H, Pip...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com