Method for refining dabigatran etexilate and method for controlling specific degradation impurities of dabigatran etexilate
A technology of dabigatran etexilate and refining method, which is applied in the field of medicine, can solve the problems of short reaction steps and no attention to the problem of product impurity content, and achieve the effects of easy operation, less solvent types and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The refining method of embodiment 1 dabigatran etexilate:
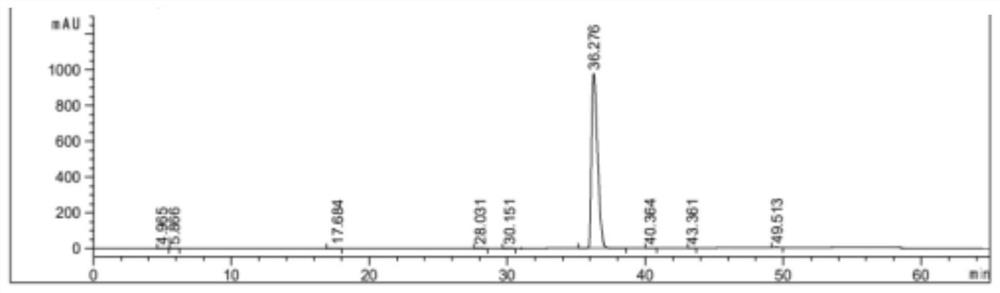
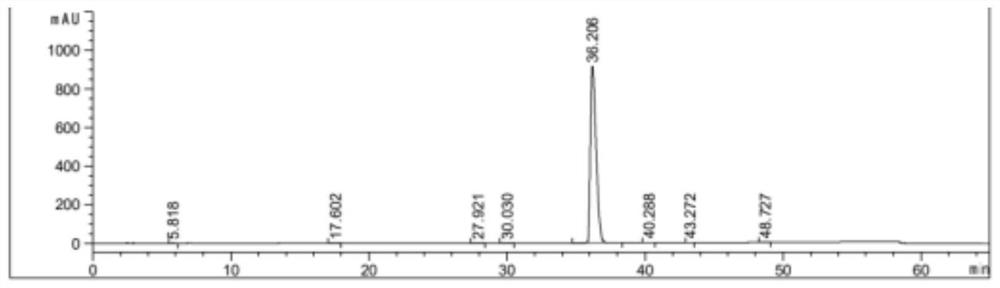
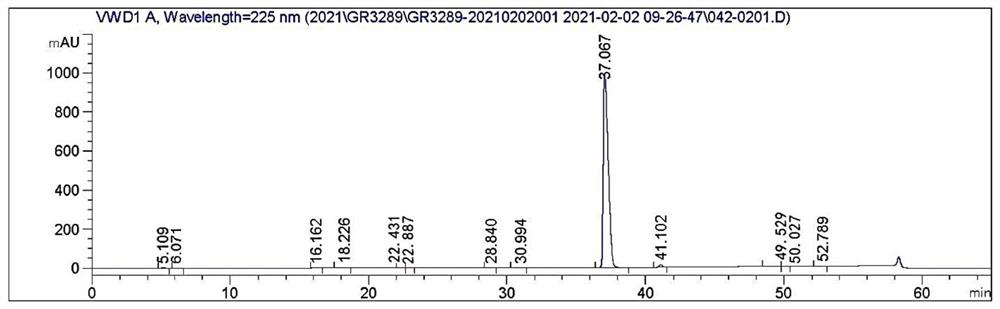
[0045] Dissolve 10g of dabigatran etexilate in 50mL of acetone, raise the temperature to dissolve, then drop the temperature and add 60mL of water, continue to cool down to 10°C and stir for 1 hour, then filter, the filter cake is washed with acetone-water mixed solution, and the dry product is dissolved after vacuum drying. In 50mL of acetonitrile, heat up and stir to dissolve, then slowly cool down to 60°C and keep stirring for 1 hour, then continue to drop to 25°C, keep stirring for 1 hour, filter, wash the filter cake with acetonitrile, and dry it in vacuum at 40°C to obtain dabigatran etexilate 8.8g, yield 75.2%, purity 99.52%.
Embodiment 2
[0046] The refining method of embodiment 2 dabigatran etexilate:
[0047] Dissolve 30g of dabigatran etexilate in 150mL of acetone, heat up to dissolve, then drop the temperature and add 300mL of water, continue to cool down to 15°C and stir for 1 hour, then filter, the filter cake is washed with acetone-water mixed solution, dried in vacuum, and then dissolved In 198mL of acetonitrile, heat up to 70°C and stir to dissolve, then slowly cool down to 50°C and keep stirring for 2 hours, then continue to drop to 20°C, keep stirring for 2 hours, then filter, wash the filter cake with acetonitrile, and dry it under vacuum at 45°C to obtain Darby Gatran etexilate 25.6g, yield 72.9%, purity 99.65%.
Embodiment 3
[0048]The refining method of embodiment 3 dabigatran etexilate:
[0049] Dissolve 40g of dabigatran etexilate in 240mL of acetone, raise the temperature to dissolve, then drop the temperature and add 240mL of water, continue to cool down to 10°C and stir for 1 hour, then filter, wash the filter cake with acetone-water mixed solution, vacuum dry and dissolve the dry product In 394mL of acetonitrile, heat up to 70°C and stir to dissolve, then slowly cool down to 55°C and keep stirring for 1 hour, then continue to drop to 20°C, keep stirring for 1 hour, then filter, wash the filter cake with acetonitrile, and dry it under vacuum at 45°C to obtain the ratio Gatran etexilate 34.7g, yield 74.2%, purity 99.67%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com