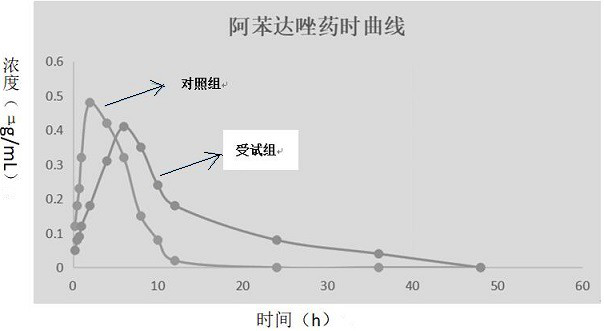
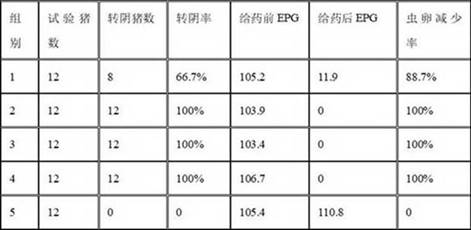
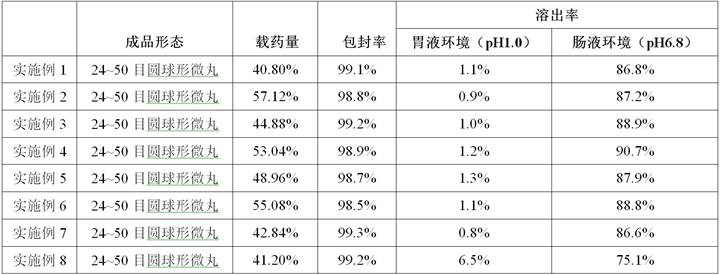
Albendazole ivermectin enteric-coated sustained-release pellet and preparation method thereof
A technology of albendazole ivermectin and sustained-release pellets, which is applied in the field of albendazole ivermectin enteric-coated sustained-release pellets and its preparation, can solve the problem of high toxicity and time-consuming albendazole , cost and other issues, to achieve the effect of good intestinal disintegration and dissolution, safety and high efficiency, and reduced dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1 Preparation of enteric-coated pellets of the present invention
[0034] Take the raw and auxiliary materials according to the following weight ratio:
[0035] The amount of raw and auxiliary materials per 100g ball core:
[0036] Albendazole 50.0g
[0037] Ivermectin 1.0g
[0038] Hypromellose 49.0g
[0039] Per 100g enteric-coated layer, the amount of excipients:
[0040] Eudragit L30D-55 Water Dispersion 42.0g
[0041] Diethyl phthalate 1.1g
[0042] Talc powder 1.9g
[0043] Purified water 55.0g;
[0044] The ratio of the core to the coating layer of the enteric-coated pellets by weight is 8:2.
[0045] The preparation method is as follows
[0046] a. Preparation of ball core: fully mix albendazole and ivermectin, add a 1.6% v / v hydroxypropyl methylcellulose aqueous solution to make a soft material, and make the soft material at a speed of 50r / min Extruded under the same conditions to make strips, and then cut and spheronized by a spheronizer w...
Embodiment 2
[0048] Embodiment 2 Preparation of enteric-coated pellets of the present invention
[0049] Take the raw and auxiliary materials according to the following weight ratio:
[0050] The amount of raw and auxiliary materials per 100g ball core:
[0051] Albendazole 70.0g
[0052] Ivermectin 1.4g
[0053] Hypromellose 28.6g
[0054] Per 100g enteric-coated layer, the amount of excipients:
[0055] Eudragit L30D-55 Water Dispersion 42.0g
[0056] Diethyl phthalate 1.1g
[0057] Talc powder 1.9g
[0058] Purified water 55.0g;
[0059] The ratio of the core to the coating layer of the enteric-coated pellets by weight is 8:2.
[0060] The preparation method is as follows
[0061] a. Preparation of ball core: fully mix albendazole and ivermectin, add a 1.6% v / v hydroxypropyl methylcellulose aqueous solution to make a soft material, and make the soft material at a speed of 50r / min Extruded under the same conditions to make strips, and then cut and spheronized by a spheronizer w...
Embodiment 3
[0063] Embodiment 3 Preparation of enteric-coated pellets of the present invention
[0064] Take the raw and auxiliary materials according to the following weight ratio:
[0065] The amount of raw and auxiliary materials per 100g ball core:
[0066] Albendazole 55.0g
[0067] Ivermectin 1.1g
[0068] Hypromellose 43.9g
[0069] Per 100g enteric-coated layer, the amount of excipients:
[0070] Eudragit L30D-55 Water Dispersion 42.0g
[0071] Diethyl phthalate 1.1g
[0072] Talc powder 1.9g
[0073] Purified water 55.0g;
[0074] The ratio of the core to the coating layer of the enteric-coated pellets by weight is 8:2.
[0075] The preparation method is as follows
[0076] a. Preparation of ball core: fully mix albendazole and ivermectin, add a 1.6% v / v hydroxypropyl methylcellulose aqueous solution to make a soft material, and make the soft material at a speed of 50r / min Extruded under the same conditions to make strips, and then cut and spheronized by a spheronizer w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap