Sirolimus gel preparation
A technology of sirolimus gel and gel preparation, applied in the field of pharmaceutical preparations, can solve the problems of affecting the absorption rate, instability, poor acid-base tolerance of sirolimus and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1 The stability of sirolimus solution with different ethanol proportions
[0026] Weigh 0.2g of sirolimus and add 10g of ethanol to dissolve, add an appropriate amount of purified water to 100g to obtain a sirolimus solution with 10% ethanol, and prepare 30%, 50%, 70%, and 100% ethanol in the same way. The sirolimus solution with different proportions of ethanol was carried out for stability setting out, and the sirolimus solution was kept away from light, and the content of sirolimus in the solution was determined according to high performance liquid chromatography (Chinese Pharmacopoeia 2020 edition general rule 0512).
[0027] Chromatographic conditions and system adaptability test: Octylsilane bonded silica gel was used as filler, methanol-water-acetonitrile (30:30:40) was used as mobile phase, detection wavelength was 277nm, column temperature was 40°C, theoretical plate The number is not less than 1500 according to the main peak of sirolimus.
[0028] ...
Embodiment 2
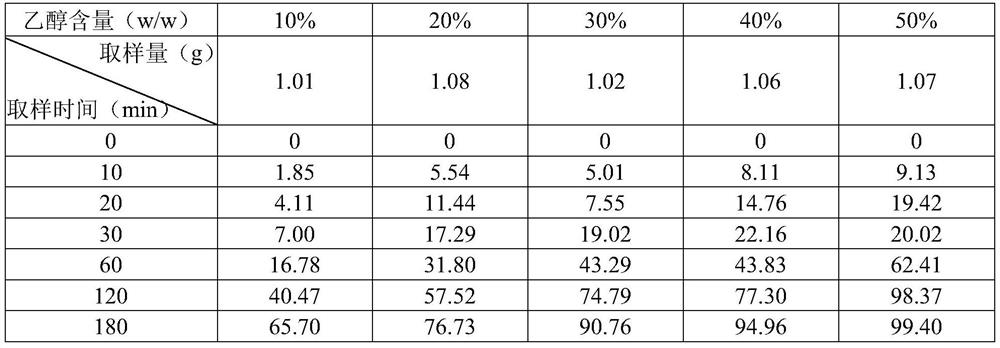
[0033] Example 2 Sirolimus Gel Stability of Different Ethanol Proportions
[0034] To prepare sirolimus gels in 10% ethanol:
[0035] Step a. Weigh 0.2g sirolimus and add to 10g ethanol for dissolving;
[0036] Step b. Weigh 1g of carbomer, add appropriate amount of purified water, fully swell overnight, and add triethanolamine to adjust the pH to 5.06;
[0037] Step c. Slowly add the sirolimus solution into the carbomer matrix, add an appropriate amount of purified water to 100 g as needed, stir until it is in a gel state, and obtain a sirolimus gel with 10% ethanol concentration, and measure the pH of the gel Value is 5.55
[0038] The sirolimus gel with 50% ethanol concentration was prepared in the same step, and the pH value of the final gel was determined to be 5.60
[0039] The sirolimus gels with different ethanol proportions were stably sampled and operated in the dark, and the content of sirolimus in the gels was determined according to high performance liquid chro...
Embodiment 3
[0052] Sirolimus gel stability under different pH value conditions of embodiment 3
[0053] Preparation of sirolimus gels at different pH:
[0054] Step a. Weigh 0.2g sirolimus and add to 50g ethanol for dissolving;
[0055]Step b. Weigh 1g of carbomer, add appropriate amount of purified water, fully swell overnight, add different amounts of triethanolamine dropwise to adjust pH, and obtain gel matrix with different pH;
[0056] Step c. Slowly add the sirolimus solution obtained in step a to the gel matrix obtained in step b, add an appropriate amount of purified water to 100 g as needed, measure the pH, add triethanolamine as needed to adjust the pH again, and finally stir until In the gel state, sirolimus gels with different pHs in the table below were obtained.
[0057] The sirolimus gels with different pH were carried out in a stable setting, operated in the dark, and the content of sirolimus in the gel was determined by high performance liquid chromatography. The result...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com