Synthesis method of three-function diagnosis and treatment integrated prodrug for prostate cancer and product thereof
A technology of prostate cancer and synthesis method, which is applied in the field of synthesis of three-function diagnosis and treatment integrated precursor drugs, can solve the problems of limiting the production and sales of PSMAI&T drugs, lack of availability, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
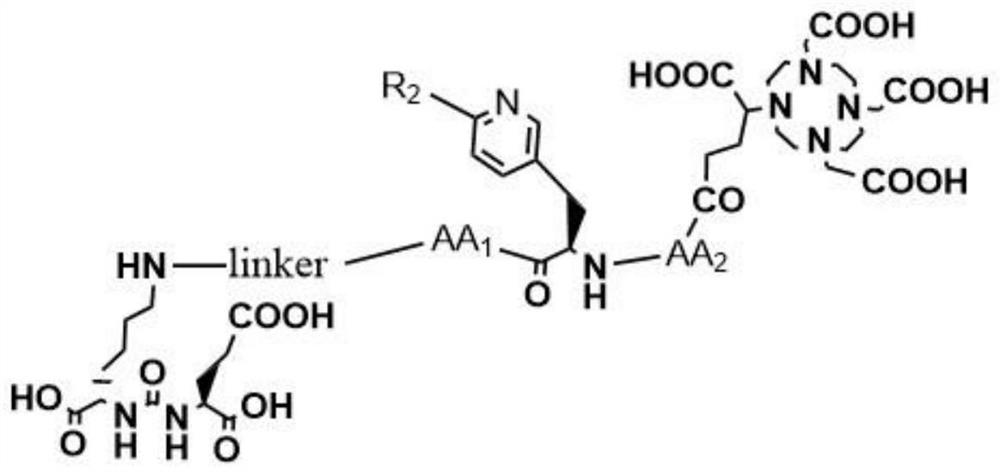
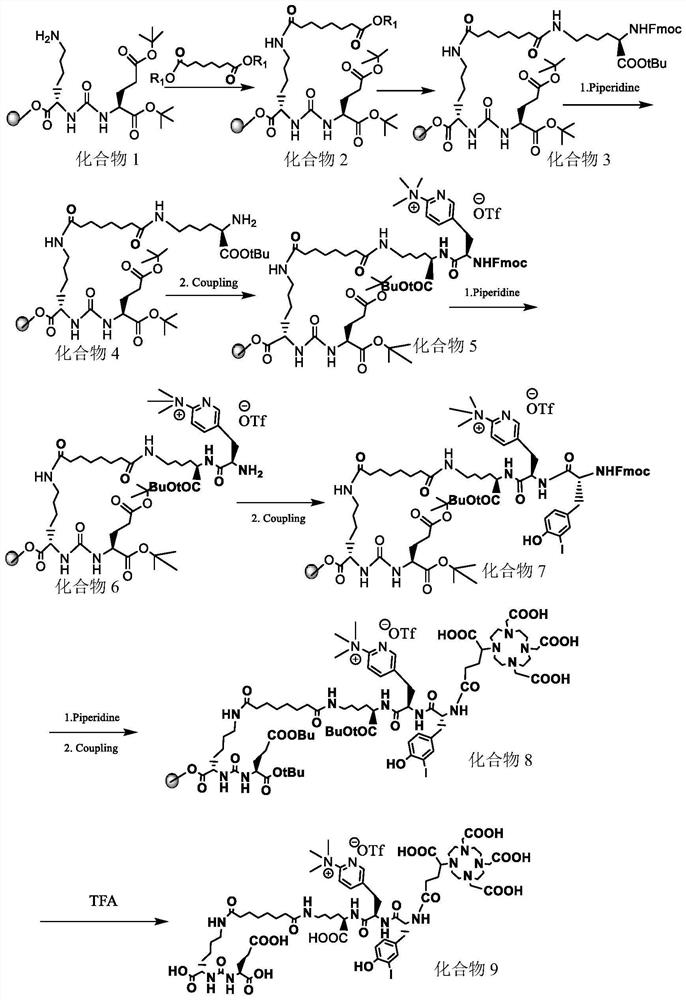
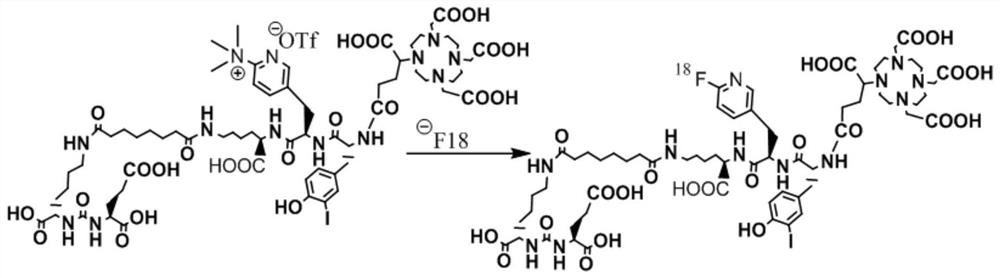
[0040] Such as Figure 1-5 Shown, a kind of synthetic method of the prodrug precursor of three-function diagnosis and treatment integration for prostate cancer, comprises the following steps:
[0041] (1) Using 1.00eq of compound 1 as a raw material, drop compound 1 into the active ester solution, suction filter, wash and dry after the stirring reaction is complete, to obtain compound 2; the solvent of the active ester solution is preferably dimethylformamide; The molar ratio of compound 1 to active ester solution is 1:1.10-2.00, the reaction temperature is room temperature, and the amino group reaction is judged to be complete through the color reaction;
[0042] (2) Put compound 2, amino acid 1, and aprotic polar solvent obtained in step (1) into a reaction flask for mixing, then add an organic base dropwise, stir and react, and obtain compound 3 by suction filtration, washing and drying;
[0043] (3) Add piperidine solution to compound 3, and obtain compound 4 after reacti...
Embodiment 1
[0058] Such as Figure 1-2 Shown, a kind of synthetic method of the prodrug precursor of three-function diagnosis and treatment integration for prostate cancer, comprises the following steps:
[0059] (1) Using compound 1 (1.00eq) as a raw material, drop compound 1 (5.08g, 2.69mmol) into 10mL active ester in dimethylformamide solution (containing active ester 1.77g, 4.02mmol), at room temperature The mixture was stirred and reacted for 3 hours, and the color reaction showed that the amino group had basically reacted completely, then suction filtered, washed and dried with dimethylformamide solution, and compound 2 was obtained;
[0060] (2) the above-mentioned compound 2, amino acid 1 (3.45g, 8.14mmol) and dimethylformamide (10mL) were added to the reaction flask, and N,N-diisopropylethylamine (1.02g, 7.89mmol) was added dropwise at room temperature, and the reaction was stirred at room temperature for 3h, Suction filtration, washing and drying with dimethylformamide to obt...
Embodiment 2
[0070] Such as Figure 1-2 Shown, a kind of synthetic method of the prodrug precursor of three-function diagnosis and treatment integration for prostate cancer, comprises the following steps:
[0071] (1) Using compound 1 (1.00eq) as raw material, drop compound 1 (4.74g, 2.51mmol) into 10mL of active ester in dimethylformamide solution (containing active ester 1.22g, 2.77mmol), at room temperature The mixture was stirred and reacted for 4 hours, and the color reaction showed that the amino group had basically reacted, then suction filtered, washed and dried with dimethylformamide solution, and compound 2 was obtained;
[0072] (2) the above-mentioned compound 2, amino acid 1 (2.13g, 5.03mmol) and dimethylformamide (10mL) were added to the reaction flask, and N,N-diisopropylethylamine (0.65g, 5.04mmol) was added dropwise at room temperature, and the reaction was stirred at room temperature for 2~ 8h, suction filtration, washing and drying with dimethylformamide to obtain com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com