Application of phosphatidylcholine in preparation of medicine for treating ulcerative colitis
A technology of ulcerative colitis and phosphatidylcholine, which is applied in the field of medicine, can solve the problems of unclear etiology of inflammatory bowel disease and difficulties in disease diagnosis and treatment, and achieve good therapeutic activity, relieve colon shortening, and relieve inflammatory infiltration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
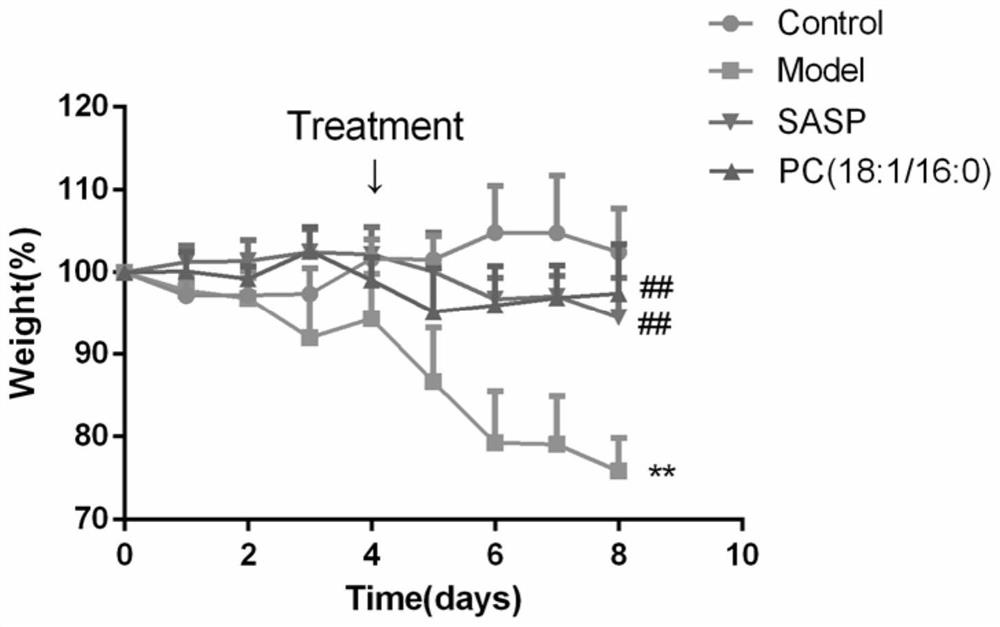
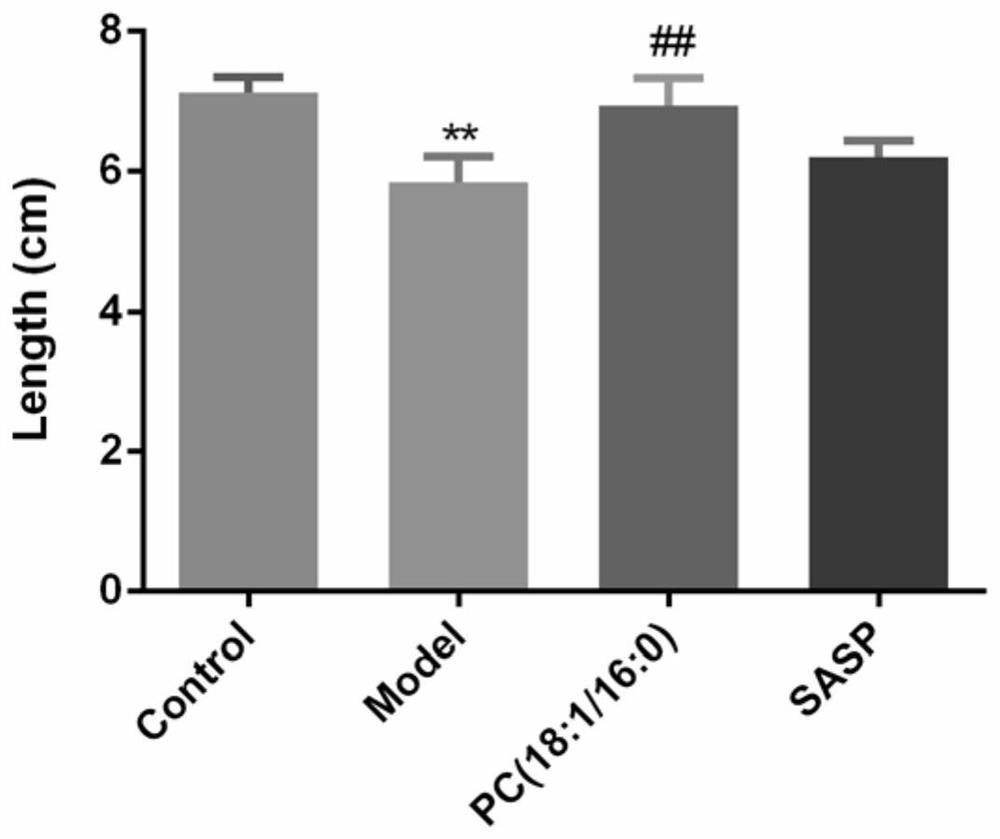
[0015] Example 1: Application research of PC (18:1 / 16:0) in treating ulcerative colitis induced by sodium dextran sulfate
[0016] Step 1: Experimental grouping (8-week-old C57 male mice, n=6)
[0017] i) Control group: After the modeling started, normal saline (0.2 mL / 20 g) was injected intraperitoneally every day, and they were allowed to eat and drink freely.
[0018] ii) Modeling group: free to eat and drink 2.5% dextran sodium sulfate aqueous solution. After the modeling started, an equal volume of normal saline (0.2 mL / 20 g) was injected intraperitoneally every day.
[0019] iii) Treatment group: free to eat and drink 2.5% dextran sodium sulfate aqueous solution. After molding, intraperitoneally inject 2mg / kg PC (18:1 / 16:0) suspension every day.
[0020] iii) Positive drug group: free to eat and drink 2.5% dextran sodium sulfate aqueous solution. After molding, 100 mg / kg sulfasalazine (SASP) suspension was administered intragastrically every day.
[0021] Step 2: Es...
Embodiment 2
[0027] Example 2: Pharmacological study of PC (18:1 / 16:0) in treating ulcerative colitis induced by sodium dextran sulfate
[0028] Step 1: Experimental grouping (8-week-old C57 male mice, n=6)
[0029] i) Control group: After the modeling started, normal saline (0.2 mL / 20 g) was injected intraperitoneally every day, and they were allowed to eat and drink freely.
[0030] ii) Modeling group: free to eat and drink 2.5% dextran sodium sulfate aqueous solution. After the modeling started, normal saline (0.2 mL / 20 g) was injected intraperitoneally every day.
[0031] iii) Treatment group: free to eat and drink 2.5% dextran sodium sulfate aqueous solution. After molding, intraperitoneally inject 2mg / kg PC (18:1 / 16:0) suspension every day.
[0032] iii) Positive drug group: free to eat and drink 2.5% dextran sodium sulfate aqueous solution. After molding, 100 mg / kg sulfasalazine (SASP) suspension was administered intragastrically every day.
[0033] Step 2: Establish a model of...
Embodiment 3
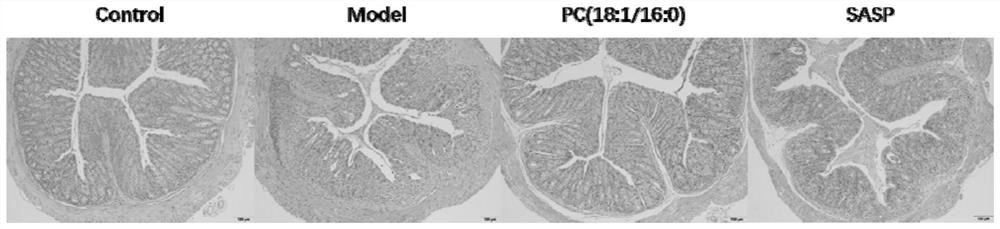
[0047] Example 3: Study on the mechanism of PC (18:1 / 16:0) in treating ulcerative colitis induced by sodium dextran sulfate
[0048] Step 1: Experimental grouping (8-week-old C57 male mice, n=6)
[0049] i) Control group: After the modeling started, normal saline (0.2 mL / 20 g) was injected intraperitoneally every day, and they were allowed to eat and drink freely.
[0050] ii) Modeling group: free to eat and drink 2.5% dextran sodium sulfate aqueous solution. After the modeling started, an equal volume of normal saline (0.2 mL / 20 g) was injected intraperitoneally every day.
[0051] iii) Treatment group: free to eat and drink 2.5% dextran sodium sulfate aqueous solution. After molding, intraperitoneally inject 2mg / kg PC (18:1 / 16:0) suspension every day.
[0052] iii) Positive drug group: free to eat and drink 2.5% dextran sodium sulfate aqueous solution. After molding, 100 mg / kg sulfasalazine (SASP) suspension was administered intragastrically every day.
[0053] Step 2: Es...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com