Xerophthalmia treatment preparation and preparation method thereof
A dry eye disease and preparation technology, applied in the field of dry eye treatment preparations and their preparation, can solve the problems of poor treatment effect of dry eye disease, inability to completely supplement ocular surface components of patients with dry eye disease, and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] A preparation method for dry eye treatment preparation, comprising the following steps:
[0038] Weigh the mucin and add water for injection to stir and dissolve, filter and sterilize through a 0.22um microporous membrane to obtain a mucin solution;
[0039] Mix sodium hyaluronate, glycerin and oil, shear and emulsify in emulsifier dissolved in water, and then homogenize in a high-pressure homogenizer to obtain an emulsified solution;
[0040] Fusing the emulsified solution with the preparation matrix, adjusting the pH of the solution to between 6.5 and 7.8, and performing high-pressure sterilization to obtain a sterile intermediate product;
[0041] The aseptic intermediate product is mixed with the mucin solution, and packaged under aseptic conditions to obtain the finished preparation.
[0042] In the above scheme, preferably, the shear rate is 500-1000 rpm, and the emulsification time is 10 min.
[0043] In the above solution, preferably, the primary valve pressur...
specific Embodiment 1
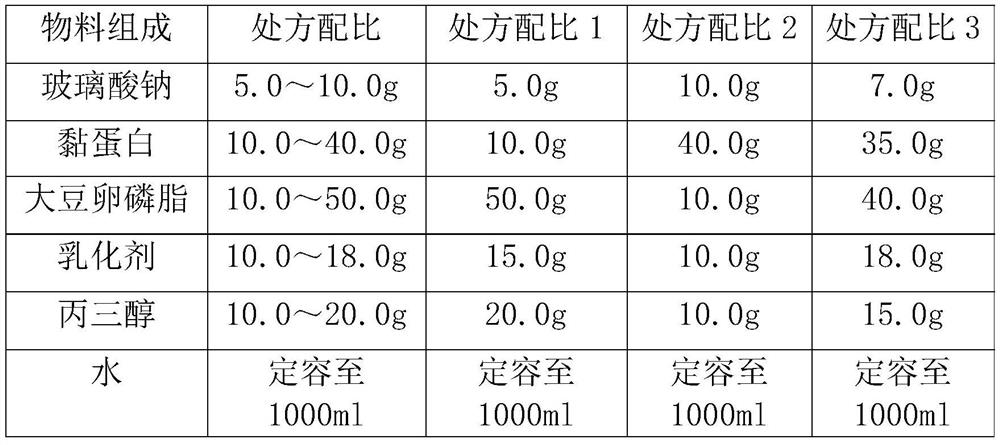
[0051]
[0052] Preparation method: a. Weigh the mucin and add 100ml of water for injection, stir and dissolve at a temperature of 30-50°C, filter and sterilize through a 0.22um microporous membrane to obtain a mucin solution;
[0053] b. Mix glycerol, sodium hyaluronate and soybean lecithin, add the aqueous solution of poloxamer 188 emulsifier, and use a high-pressure homogenizer to shear and emulsify, wherein the shear rate is 500-1000rpm, and the emulsification time is 10min ;Adjust the primary valve pressure of the high-pressure homogenizer to 400-800bar, and homogenize for 5-10 minutes to obtain a uniformly dispersed emulsified solution;
[0054] c. After the high-pressure homogenization, adjust the pH of the emulsified solution to 6.5-7.8, and place it at 121°C for 20 minutes for autoclaving;
[0055] d. Evenly disperse the mucin solution into the above solution, dilute to 1000ml with water for injection, and seal under aseptic conditions to obtain a single-dose eye d...
specific Embodiment 2
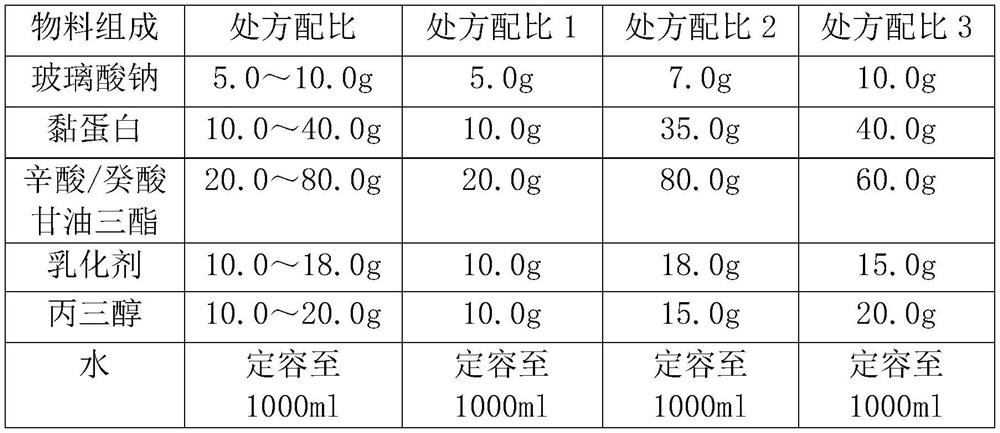
[0057]
[0058] Preparation method: a. Weigh the mucin and add 100ml of water for injection, stir and dissolve at a temperature of 30-50°C, filter and sterilize through a 0.22um microporous membrane to obtain a mucin solution;
[0059] b. Mix glycerol, sodium hyaluronate and caprylic / capric triglyceride, add the aqueous solution of poloxamer 188 emulsifier, and shear and emulsify for 10 minutes at a shear rate of 500-1000 rpm; The pressure of the primary valve of the quality machine is 400-800bar, homogenize for 5-10 minutes, and obtain a uniformly dispersed emulsified solution;
[0060] c. After the high-pressure homogenization, adjust the pH of the emulsified solution to 6.5-7.8, and place it at 121°C for 20 minutes for autoclaving;
[0061] d. Evenly disperse the mucin solution into the above solution, dilute to 1000ml with water for injection, and seal under aseptic conditions to obtain a single-dose eye drop.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com