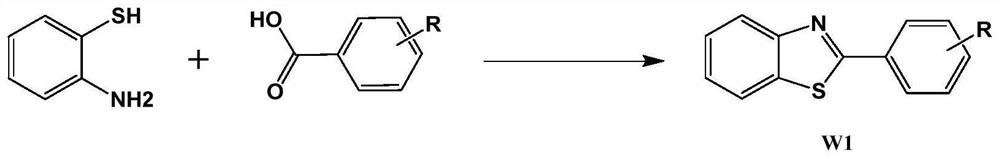
Preparation method of 2-phenylbenzothiazole derivative
A technology of derivatives, phenylbenzene, is applied in the field of preparation of 2-phenylbenzothiazole derivatives, and can solve the problems of large raw material pollution, long reaction steps, complicated post-processing and the like of 2-phenylbenzothiazole derivatives , to achieve the effect of simple and easy synthesis operation, low energy consumption and high atomic utilization rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Add 480ml polyphosphoric acid and 120ml phosphoric acid to a 1000ml reactor, heat up to 80°C, start stirring, then add (0.384mol, 48g) o-aminothiophenol and stir for half an hour, then add (0.384mol, 77g) p-bromobenzoic acid ; Heating up to 150°C, reacting for 1 hour, cooling down to 80°C, adding to 1200ml water to quench for 1 hour, filtering with suction funnel to obtain solid crude product, dissolving the solid crude product in ethyl acetate, passing through a silica gel column, and concentrating to obtain the fine product Product 2-(4-bromophenyl)benzothiazole, HPLC=99.2%, 102.5g yield 92%.
Embodiment 2
[0045] Add 480ml polyphosphoric acid and 120ml phosphoric acid to a 1000ml reactor, heat up to 80°C, start stirring, then add (0.384mol, 48g) o-aminothiophenol and stir for half an hour, then add (0.384mol, 77g) m-bromobenzoic acid ; Heating up to 160°C, reacting for 2 hours, cooling down to 90°C, adding to 1200ml water to quench for 1 hour, filtering with suction funnel to obtain solid crude product, dissolving the solid crude product in ethyl acetate, passing through a silica gel column, and concentrating to obtain the fine product Product 2-(3-bromophenyl)benzothiazole, HPLC=99.3%, 91.3g, yield 85%.
Embodiment 3
[0047]Add 480ml polyphosphoric acid and 120ml phosphoric acid to a 1000ml reactor, heat up to 80°C, start stirring, then add (0.384mol, 48g) o-aminothiophenol and stir for half an hour, then add (0.768mol, 105.2g) p-aminobenzene Formic acid; heat up to 170°C, react for 3 hours, cool down to 80°C, add to 1200ml water to quench for 1 hour, detect the raw material o-aminothiophenol through liquid phase to complete the reaction but there is a large excess of p-aminobenzoic acid, filter with suction The solid crude product was obtained by suction filtration through a funnel. The solid crude product was dissolved in ethyl acetate and passed through a silica gel column. After concentration, the crude product had a purity of 94.3%, and then recrystallized with ethanol to obtain the product 2-(4-aminophenyl)benzothiazole, HPLC=99.0 %, 45.1g yield 52%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com