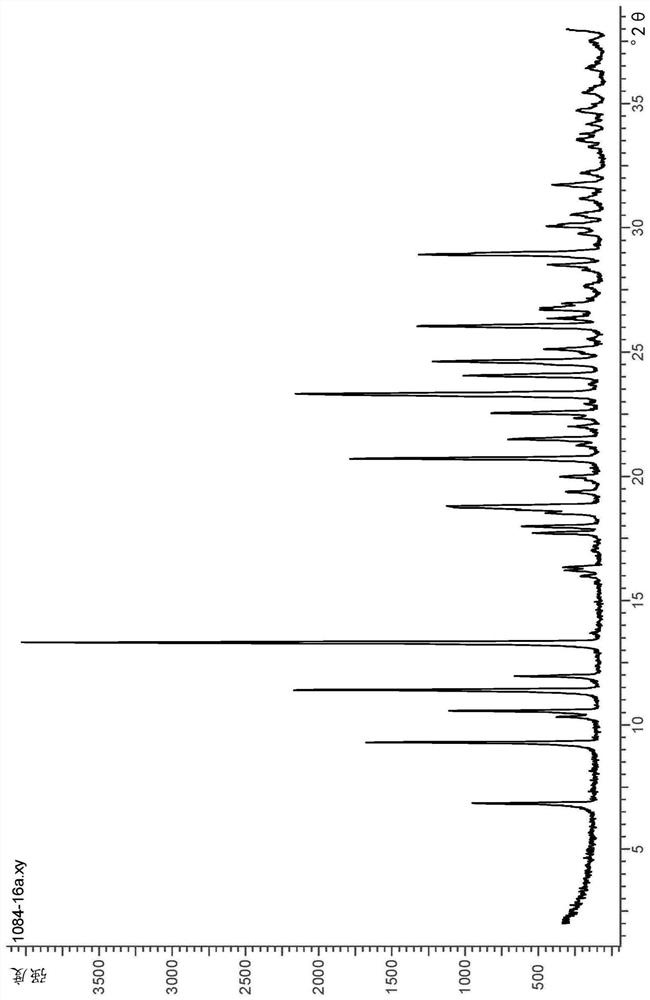
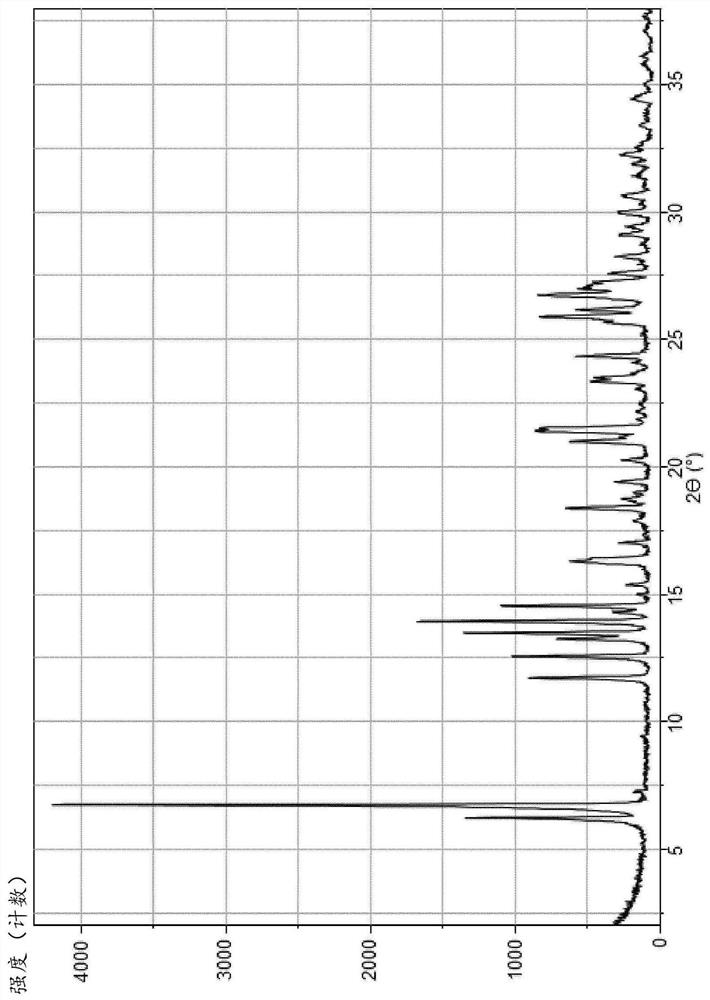
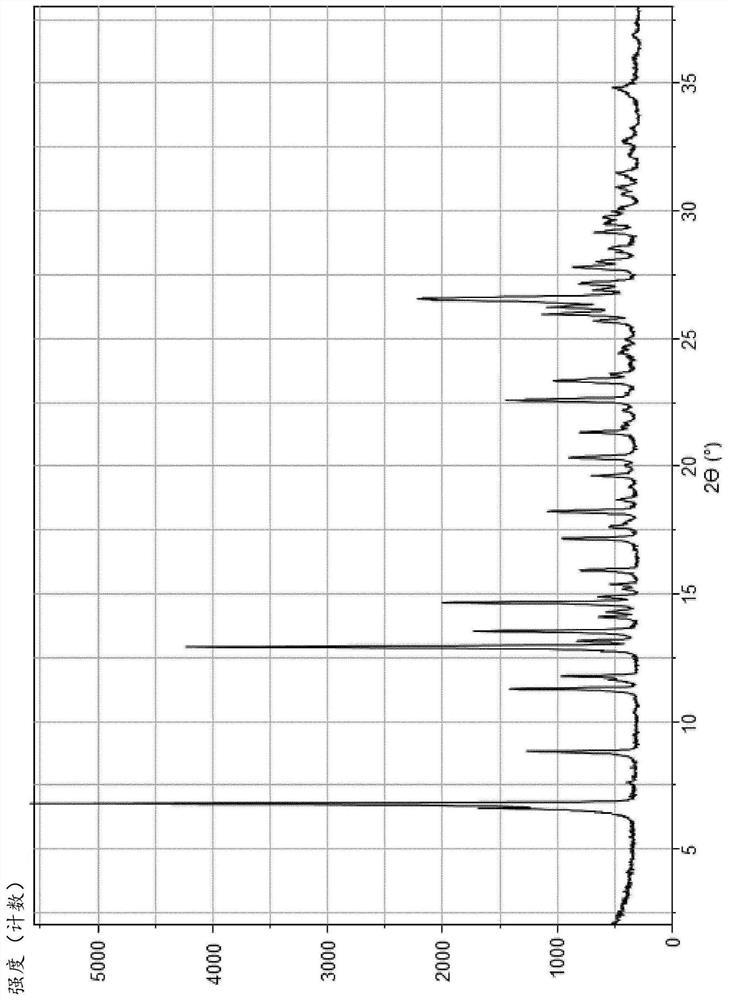
Monohydrate of rogaratinib hydrochloride and solid states thereof
A technology of monohydrate and compound, which is applied in the field of rogatinib hydrochloride monohydrate and its solid state, and can solve problems such as difficulty in large-scale treatment of dihydrochloride
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0108] Process for preparing hydrogen chloride monohydrate (III)
[0109] One aspect of the present invention pertains to a process for preparing the monochloride salt (II), more particularly its crystalline monohydrate form having a chemical composition as in formula (III).
[0110]
[0111] A general advantage of the present invention is that it provides compounds (I) and (III) in satisfactory yields with extremely low impurity levels, which is consistent with late-stage clinical development or market availability for APIs, as compared to prior art processes. requirements. The process according to the invention can be carried out without the use of chromatographic purification steps. In addition, the prior art processes have certain drawbacks that hinder the application of industrial scale production, such as process safety issues, product breakdown and increased formation of impurities due to increased processing time at scale-up, and due to high dilution The resulti...
Embodiment 1
[0390] Example 1: tert-butyl 1H-pyrrol-1-ylcarbamate (XI)
[0391]
[0392] First 1045 kg of dioxane, 350 kg of tert-butyl hydrazinecarboxylate and 420 kg of 2,5-dimethoxytetrahydrofuran were charged to the stirred reaction vessel, followed by 21.4 kg of pyridine hydrochloride and 343 kg of pyridine. The transfer line was flushed with a total of 40 L of dioxane. The reaction mixture was heated to 102±3°C for 7h and 1620L of solvent was distilled off. The batch was cooled to 25±5°C and 1750 kg of water was fed into the reactor over 1 h, maintaining the temperature at 22±3°C. The mixture was stirred for at least 30 min, then 270 kg of di-n-butyl ether were added and the temperature was adjusted to 10±3°C. The suspension was stirred for 2 h and then separated into two parts on a centrifuge. Each portion was washed with a mixture of 67 kg of di-n-butyl ether and 60 kg of heptane, followed by 175 L of heptane. The product was dried at 60°C to give 288 kg of tert-butyl 1H-p...
Embodiment 2
[0394] Example 2: tert-butyl (2-cyano-1H-pyrrol-1-yl)carbamate (XII)
[0395]
[0396] 190 kg of tert-butyl 1H-pyrrol-1-ylcarbamate (XII) and 678 kg of dry DMF were stirred in a reaction vessel at 22±3° C. until the product dissolved. The batch was cooled to 0±3°C and 163 kg of chlorosulfonyl isocyanate was added slowly over a minimum period of 2.5 h, keeping the mixture at this temperature. The mixture was stirred for an additional hour. In a second stirred reaction vessel, a solution of 272 kg of ammonium bicarbonate in 2660 kg of water was provided and the reaction mixture was transferred to this solution by maintaining the temperature below 25°C. The first reactor and transfer lines were flushed with 20L of DMF followed by 20L of water.
[0397] The mixture was continuously stirred for 2 h at 22±3°C, and the crude product was separated into two parts on a centrifuge, each part was washed twice with 760 L of water.
[0398] In a stirred reaction vessel, the crude pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com