Method for catalytically synthesizing ethyl cinnamate by using microporous molecular sieve
A technology of microporous molecular sieve and ethyl cinnamate, applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylic acid esters, etc., can solve the problems of non-renewable catalytic activity, expensive production cost of catalysts, difficult application, etc., to achieve Target product selectivity solution, target product selectivity is high, and the effect of reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] A. all add 1.0 g cinnamic acid and 10.0 g ethanol in three lining polytetrafluoroethylene reactors, obtain cinnamic acid ethanol solution;
[0017] b. Add 0.5 g H-ZSM-5 molecular sieve, 0.5 g H-Y molecular sieve and 0.5 g H-β molecular sieve to the ethanol solution of cinnamic acid, respectively, at 150 ℃, 160 ℃, 170 ℃, 180 ℃, 190 ℃ Reaction 8h under the reaction temperature;
[0018] c. The reaction product obtained in step b is subjected to on-line analysis by a chromatograph after vacuum distillation, and is determined to be ethyl cinnamate, and the conversion rate and selectivity of ethyl cinnamate are shown in Table 1.
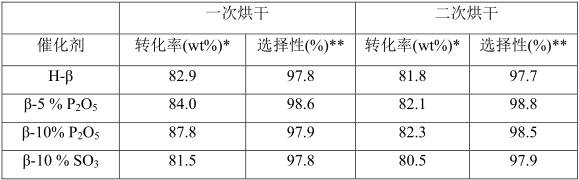
[0019] Table 1
[0020]
[0021] Note *: (result after chromatographic normalization calculation); **: (result after chromatographic normalization calculation)
Embodiment 2
[0023] A. all add 1.0 g cinnamic acid and 10.0 g ethanol in three lining polytetrafluoroethylene reactors, obtain cinnamic acid ethanol solution;
[0024] b. Add 0.5 g of cinnamic acid ethanol solution and name it β-5% P 2 o 5 , β-10% P 2 o 5 or β-10 % SO 3 The modified microporous molecular sieve was reacted at 180 ℃ for 8 h;
[0025] The β-5 % P 2 o 5 , β-10% P 2 o 5 The preparation method is: impregnating strip-shaped H-β molecular sieves in NH 4 h 2 PO 4 solution for 12 h, the solid was dried at 100 °C, and then calcined at 550 °C for 4 h in an air atmosphere to obtain β-5 % P 2 o 5 , β-10% P 2 o 5 , the NH 4 h 2 PO 4 2H 2 The concentration of O is represented by P 2 o 5 The mass ratio to strip β-type molecular sieve is 5.0%, 10.0% shall prevail;
[0026] β-10% SO 3 The preparation method is: impregnating strip-shaped H-β molecular sieves in (NH 4 ) 2 SO 4 solution for 12 h, the solid was dried at 100 °C, and then calcined at 550 °C for 4 h in an a...
Embodiment 3
[0032] A. all add 1.0 g cinnamic acid and 10.0 g ethanol in six lining polytetrafluoroethylene reactors, obtain cinnamic acid ethanol solution;
[0033] b. Add 0.025 g, 0.065 g, 0.125 g, 0.250 g, 0.750 g and 1.000 g of H-β molecular sieve to the ethanol solution of cinnamic acid, and react at a temperature of 180 °C for 8 h;
[0034] c. The reaction product obtained in step b is subjected to on-line analysis by a chromatograph after vacuum distillation, and is determined to be ethyl cinnamate, and the conversion rate and selectivity of ethyl cinnamate are shown in Table 3.
[0035] table 3
[0036]
[0037] Note *: (result after chromatographic normalization calculation); **: (result after chromatographic normalization calculation)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com