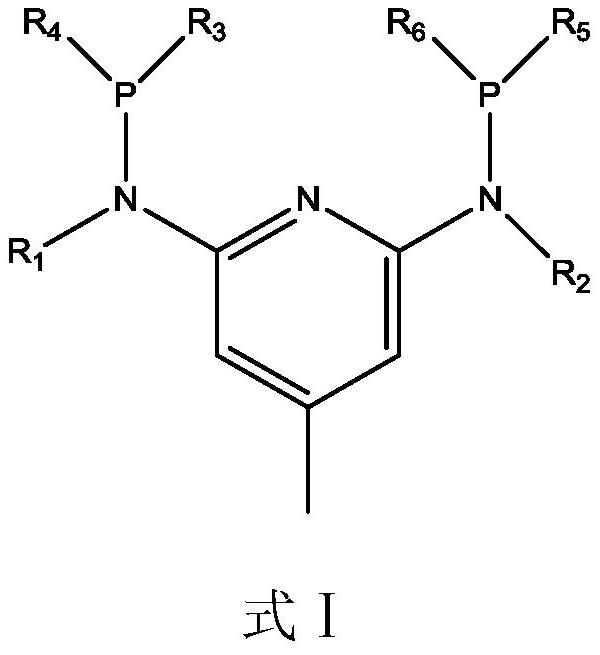
Ligand based on pyridine structure and preparation method thereof, and supported catalyst based on pyridine structure and preparation method and application thereof
A technology of pyridine and ligands, which is applied in ethylene oligomerization, preparation, ligands based on pyridine structure and its preparation, and the field of supported catalysts based on pyridine structure, which can solve the problems of difficult equipment blockage and avoid equipment blockage , high target product selectivity, and the effect of reducing the probability of clogged pipelines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] The preparation method of ligand (L1):
[0088] 1) First, heat 2,6-dibromo-4-methylpyridine (105.5mmol) and methylamine aqueous solution (211mmol) at 180°C for 5h, filter after cooling, add water (400ml) and dichloromethane ( 200ml) extraction, remove dichloromethane under vacuum conditions, the obtained product is subjected to column chromatography (height-to-diameter ratio is 2, residence time is 2min purification), and the eluent phase is ethyl acetate and n-hexane with a volume ratio of 1:1 mixed solvent, and the product was obtained after drying.
[0089] The product (80mmol) was dissolved in acetonitrile (100ml) solvent, then n-butyllithium (160mmol) was added at -80°C, reacted for 3h, the temperature was raised to -5°C and diphenylphosphine chloride (160mmol) was added, and the reaction After 1h, the reaction solution was purified by column chromatography (height-to-diameter ratio: 2, residence time: 2min), the eluting phase was a mixed solvent of ethyl acetate ...
Embodiment 2
[0096] Ligand (L2) differs from the preparation method in Example 1 in that:
[0097]1) First, heat 2,6-dibromo-4-methylpyridine (105.5mmol) and isopropylamine (232.1mmol) at 185°C for 6h, filter after cooling, add water (600ml) and dichloromethane ( 200ml) extraction, remove dichloromethane under vacuum conditions, the obtained product is subjected to column chromatography (height-to-diameter ratio is 2, residence time is 2min purification), and the eluent phase is ethyl acetate and n-hexane with a volume ratio of 1:1 mixed solvent, and the product was obtained after drying.
[0098] Dissolve the product in acetonitrile (80mmol) solvent, then add n-butyllithium (176mmol) at -75°C, react for 4h, heat up to -3°C and add diphenylphosphine chloride (176mmol), react for 2h, react The solution was purified by column chromatography (height-to-diameter ratio: 2, residence time: 2 min), and the eluting phase was a mixed solvent of ethyl acetate and n-hexane at a ratio of 1:1. Ligand ...
Embodiment 3
[0105] Ligand (L3) differs from the preparation method in Example 1 in that:
[0106] 1) First, heat 2,6-dibromo-4-methylpyridine (105.5mmol) and tert-butylamine (263.8mmol) at 190°C for 8h, filter after cooling, add water (800ml) and dichloromethane (200ml ) extraction, remove dichloromethane under vacuum condition, the product obtained carries out column chromatography (height-diameter ratio is 2, and retention time is 2min purification), and eluting phase is the ethyl acetate and normal hexane that volume ratio is 1:1 The solvents were mixed and dried to obtain the product.
[0107] Dissolve the product in acetonitrile (80mmol) solvent, then add n-butyllithium (192mmol) at -70°C, react for 5h, heat up to 0°C, add diphenylphosphine chloride (192mmol), react for 3h, the reaction solution Purified by column chromatography (height-to-diameter ratio: 2, residence time: 2 min), the eluting phase was a mixed solvent of ethyl acetate and n-hexane at 1:1, and the ligand L3 was obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com