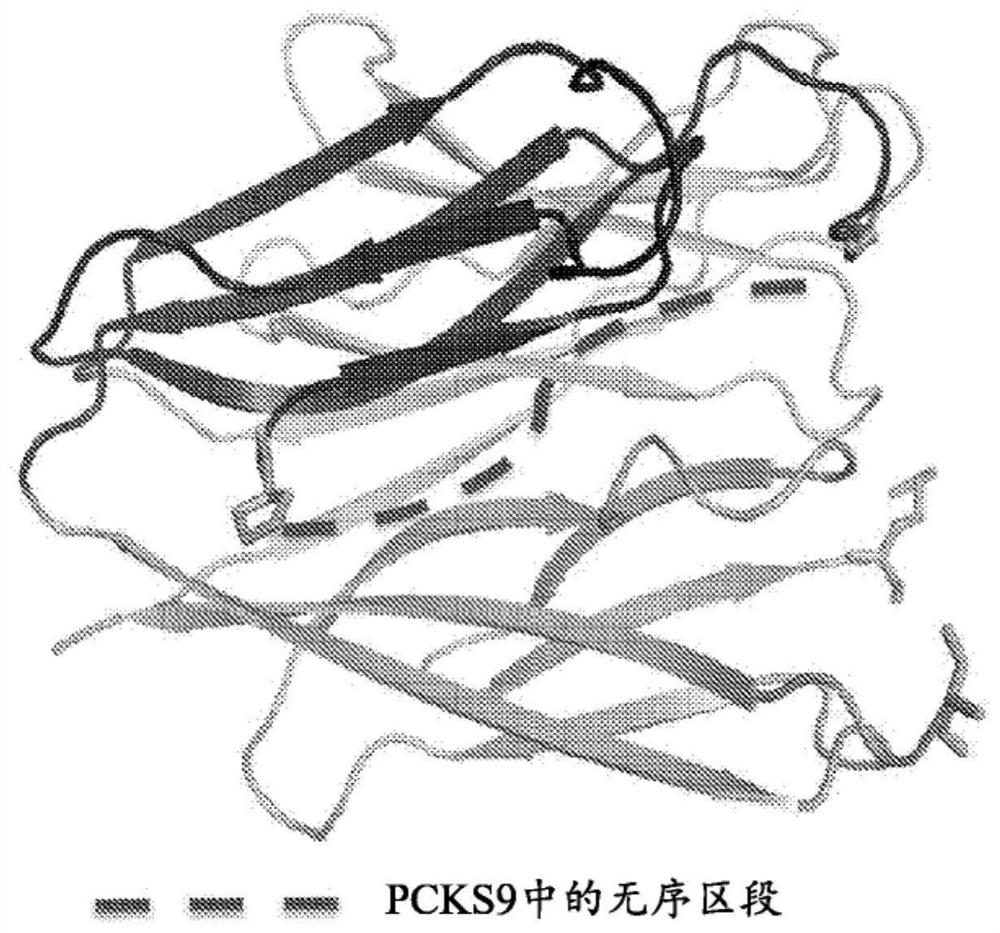
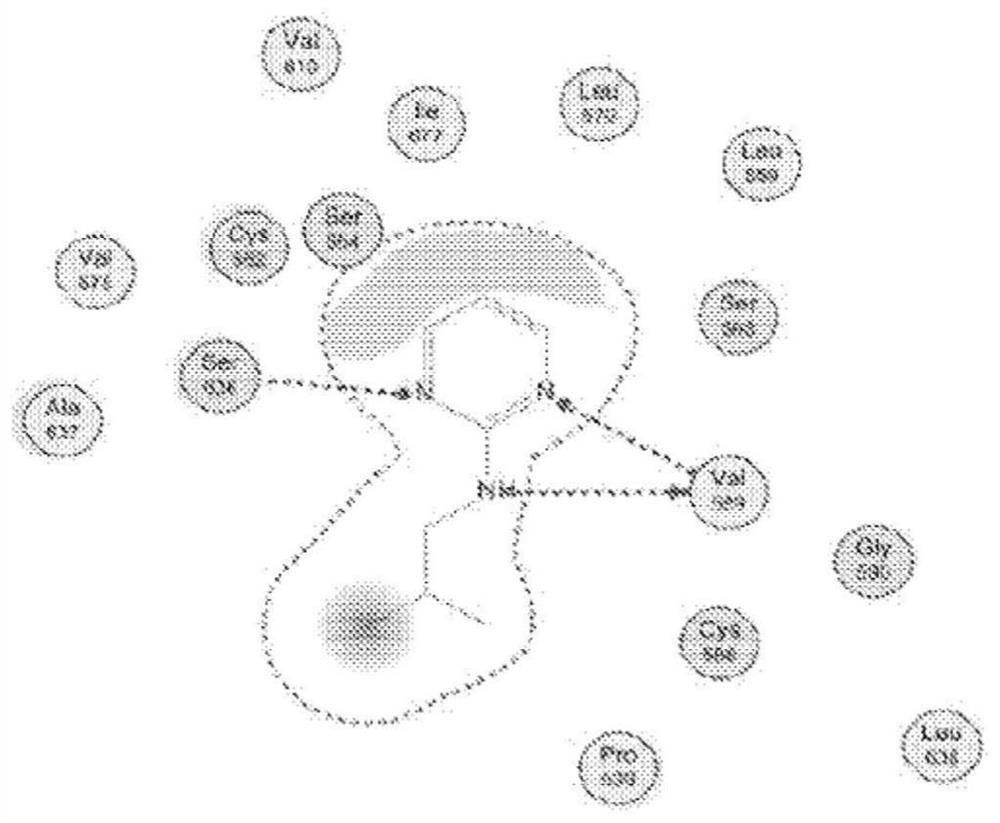
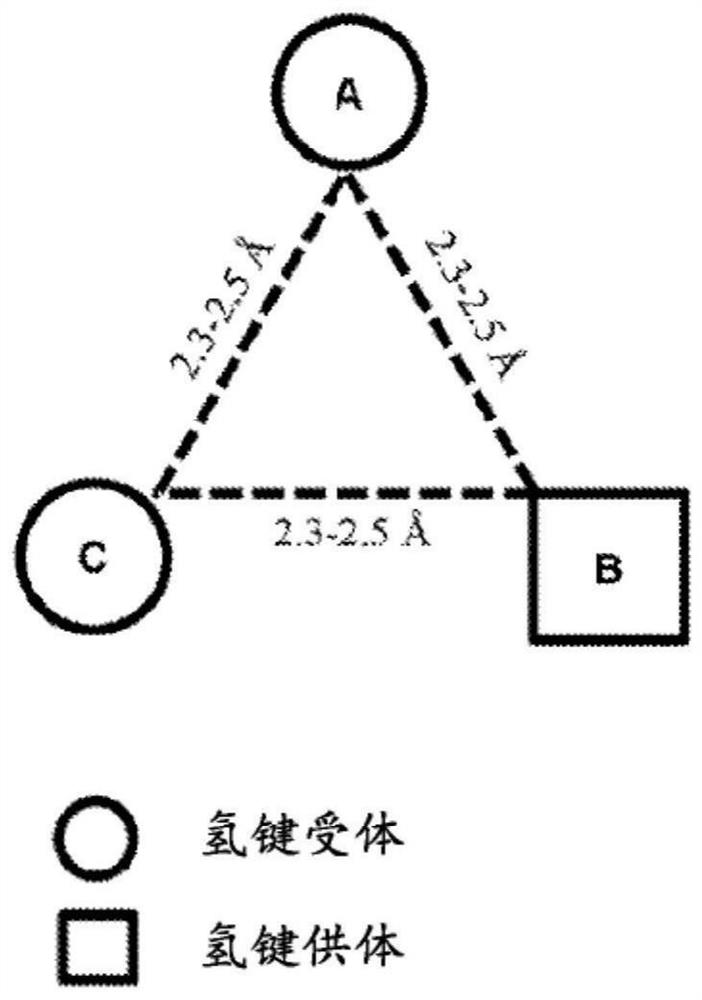
Pcsk9 inhibitors and methods of use thereof
A technology of inhibitors and residues, applied in the field of PCSK9 inhibitors and its use, can solve problems such as allergic reactions and harmful immune responses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0268] Examples of compounds of formula (I) or pharmaceutically acceptable salts thereof that have useful biological activities are listed in Tables 1-10. 1 H NMR spectroscopy was performed on a Varian MR-400 mass spectrometer operating at 400 MHz (proton frequency) equipped with: self-shielded z-gradient coil 5 mm 1H / nX reverse detection broadband probe, deuterium digital channel lock unit, with transmitter bias A frequency-shifted quadrature digital detection unit. Chemical shifts are reported as delta values in ppm relative to trimethylsilane (TMS) as internal standard. Coupling constants (J values) are given in Hertz (Hz) and diversity is reported using the following abbreviations (s=singlet, d=doublet, t=triplet, q=quartet, m=multiplet, br=broad, nd=not determined).
[0269] A. Analysis method
[0270] Method 1 (Acid FA)
[0271] UPLC settings
[0272] Solvent: -A water (high purity via PureLab Option unit) with 0.1% formic acid
[0273] B Acetonitrile with 0.1%...
example 302B
[0821] A solution of Example 302A (6.77 g, 21 mmol) in THF (200 mL) was cooled to -65 °C under N2 atmosphere. MeMgBr (21 mL, 63.1 mmol, 3M in THF) was added dropwise, then stirred at -65 °C for 0.5 h. The reaction was warmed to room temperature for 2 h, quenched by the addition of water (200 mL). After extraction with EtOAc (200 mL x 2), the combined organic layers were dried over anhydrous magnesium sulfate and concentrated under reduced pressure. The residue was purified by silica gel chromatography (eluting with petroleum ether / EtOAc = 3 / 1) to give the desired product Example 302B (4 g, 56% yield) as a yellow oil. LCMS[M+H] + =339
[0822] Step 2: Instance 302C
[0823] To the reactor, a suspension of Pd / C (400 mg) catalyst was introduced into a solution of Example 302B (4 g, 0.012 mmol) in MeOH (40 mL). The vessel was purged with nitrogen, then hydrogen, and the reaction mixture was stirred at room temperature for 18 h. Consumption of starting material was detected b...
example 303
[0846] To Example 303G (563 mg, 1.14 mmol) and K at room temperature 2 CO 3 (940 mg, 6.82 mmol) in DMF (10 mL) was added Example 301A (183 mg, 1.14 mmol). The reaction was then heated to 60 °C and stirred for 16 h. TLC showed that the starting material was consumed. The reaction mixture was cooled to room temperature and diluted with EtOAc (30 mL) and washed with water (20 mL). The aqueous layer was extracted with EtOAc (20 mL x 4), and the combined organic layers were washed with brine (20 mL x 2), washed with Na2SO 4 Dry, filter and concentrate. The residue was purified by silica gel chromatography (eluting with petroleum ether / EtOAc = 3 / 1 to 1 / 9) to provide the crude product, which was purified by preparative HPLC to provide the product Example 303 as a pale yellow solid ( 75.2 mg, yield: 12.93%). LCMS[M+H] + =511
[0847] 1H NMR (400MHz, CDCl3) δ 8.37(s, 2H), 7.96(s, 1H), 7.66-7.58(m, 2H), 6.82(s, 1H), 5.87(s, 1H), 3.83-3.73(m , 5H), 3.59-3.44 (m, 5H), 3.01 (s, 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com