Method for synthesizing tricyclic skeleton 2-pyridone and 2-pyridine imine compounds
A technology of pyridine imines and compounds, applied in the field of chemical synthesis, which can solve problems such as lack of synthesis methods, cumbersome operations, and complicated raw materials, and achieve the effects of no catalyst, high reaction yield, and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Synthetic tricyclic skeleton 2-pyridone compound
[0042]
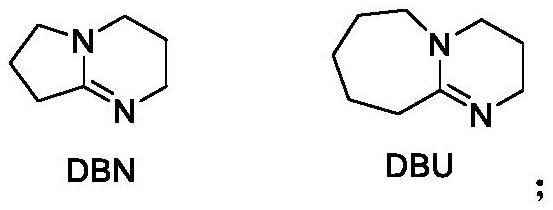
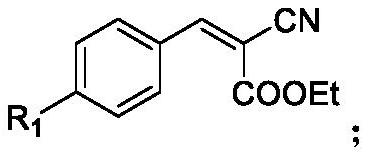
[0043] Under nitrogen protection, DBN (0.2 mmol) was added to 2-cyano-3-phenylrokylene (0.4 mmol) of acetonitrile solution (1 mL), stirred at 120 ° C for 4 h, and after the reaction, cooled to room temperature; Filtration, methanol and n-hexane recrystallization (methanol: n-hexane = 1: 5), the product was 42.5 mg, the yield was 77%, the product was a yellow solid, melting point:> 289 ° C.
[0044] IR (ATR): 3675, 2973, 2794, 1700, 1614, 1557, 1538, 1495, 1440, 1392, 1366, 1308, 1273, 1188, 764, 740, 708, 670cm -1 .
[0045] 1 H NMR (400MHz, DMSO-D 6 : Δ7.54-7.39 (m, 5H), 3.83-3.75 (m, 2H), 3.72 (T, J = 8.3Hz, 2H), 3.32-3.29 (T, 2H), 2.76 (T, J = 8.2 Hz, 2H), 2.11-1.98 (m, 2H).
[0046] 13 C NMR (100MHz, DMSO-D 6 : δ161.2, 154.9, 150.2, 136.4, 129.4, 129.0, 128.3, 12.4, 102.4, 78.4, 51.8, 41.9, 38.1, 24.1, 19.6.
[0047] HRMS (ESI): M / Z: Calcd for C 17 Hide 16 N 3 O + [M + h] + : 278.1288; Found: 278...
Embodiment 2
[0048] Example 2: Synthetic tricyclic skeleton 2-pyridone compound
[0049]
[0050] Under nitrogen protection, DBN (0.2 mmol) was added to 2-cyano-3- (4-methoxyphenyl) acrylate (0.4 mmol) of acetonitrile solution (1 mL), stirred at 120 ° C for 4 h, After the reaction was completed, cooled to room temperature; concentrated, the column chromatography was separated (ethyl acetate: methanol = 5: 1), the product was 54.4 mg, the yield was 89%, the product was a yellow solid, the melting point was 224-225 ° C.
[0051]IR (ATR): 3547,2913,2190,1711,1614,1535,1510,1459,1418,1392,1372, 1307,1278,1252,1175,1117,1025,973,891,842,771,755,725,700,655cm -1 .
[0052] 1 H NMR (400MHz, CDCL 3 ): Δ7.38 (d, J = 8.6Hz, 2H), 6.93 (d, J = 8.6Hz, 2H), 3.95-3.89 (m, 2H), 3.83 (s, 3H), 3.73 (t, J = 8.4Hz, 2H), 3.33 (t, J = 5.8Hz, 2H), 2.89 (t, J = 8.4Hz, 2H), 2.16-2.07 (m, 2H).
[0053] 13 C NMR (100MHz, CDCL 3 ): Δ161.5,160.2,154.2,151.6,129.6,127.9,119.8,113.9,101.3,81.0,55.3,51.9,42.1,37.9,24.7,19....
Embodiment 3
[0055] Example 3: Synthesis of tricyclic skeleton 2- pyridones
[0056]
[0057] Under nitrogen, the DBN (0.2mmol) was added to 2-cyano-3- (4-chlorophenyl) (1 mL) and stirred for 4h at 120 ℃ ethyl acrylate (0.4 mmol) of acetonitrile, the reaction after cooling to room temperature; concentration, separation with column chromatography (ethyl acetate: methanol = 5: 1) to give product 35mg, 56% yield, the product as a yellow solid, mp 237-238 ℃.
[0058] IR (ATR): 3545,2912,2194,1617,1554,1534,1488,1406,1371,1307,1279, 1187,1151,1108,1089,1043,1009,977,891,833,768,754,700,651cm -1 .
[0059] 1 H NMR (400MHz, CDCL 3 ): Δ7.42-7.32 (m, 4H), 3.97-3.89 (m, 2H), 3.75 (t, J = 8.4Hz, 2H), 3.35 (t, J = 5.8Hz, 2H), 2.86 (t, J = 8.4Hz, 2H), 2.19-2.08 (m, 2H).
[0060] 13 C NMR (100MHz, CDCL 3 ): Δ161.2,154.3,150.7,135.3,134.1,129.5,128.8,119.3,101.4,80.8,51.9,42.1,37.9,24.3,19.8.
[0061] HRMS (ESI): m / z: calcd for C 17 Hide 15 CLN 3 O + [M + h] + : 312.0898; found: 312.0885.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com